Shop for Plans
Shop for your own coverage
Plans through your employer
Learn about the medical, dental, pharmacy, behavioral, and voluntary benefits your employer may offer.
Learn
Looking for Medicare coverage?
Childhood Acute Lymphoblastic Leukemia Treatment (PDQ®): Treatment - Health Professional Information [NCI]
General Information About Childhood Acute Lymphoblastic Leukemia (ALL)
Cancer in children and adolescents is rare, although the overall incidence of childhood cancer, including ALL, has been slowly increasing since 1975.[
Incidence
ALL, the most common cancer diagnosed in children, represents approximately 25% of cancer diagnoses among children younger than 15 years.[
A sharp peak in ALL incidence is observed among children aged 1 to 4 years (81 cases per 1 million per year), with rates decreasing to 24 cases per 1 million by age 10 years.[
The incidence of ALL appears to be highest in American Indian or Alaska Native children and adolescents (65.9 cases per 1 million) and Hispanic children and adolescents (48 cases per 1 million).[
Anatomy
Childhood ALL originates in the T and B lymphoblasts in tissues with hematopoietic progenitor cells, such as the bone marrow and thymus (see Figure 1). 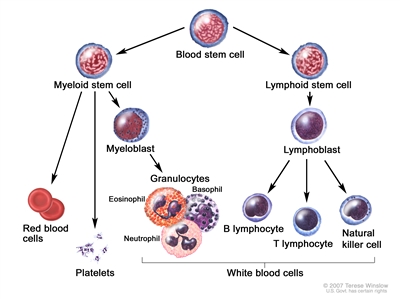
Figure 1. Blood cell development. Different blood and immune cell lineages, including T and B lymphocytes, differentiate from a common blood stem cell.
Marrow involvement of acute leukemia as seen by light microscopy is defined as follows:
- M1: Fewer than 5% blast cells.
- M2: 5% to 25% blast cells.
- M3: Greater than 25% blast cells.
Almost all patients with ALL present with an M3 marrow.
Morphology
In the past, ALL lymphoblasts were classified using the French-American-British (FAB) criteria as having L1, L2, or L3 morphology.[
Most cases of ALL that show L3 morphology express surface immunoglobulin (Ig) and have a MYC gene translocation identical to those seen in Burkitt lymphoma (i.e., t(8;14)(q24;q32), t(2;8)) that join MYC to one of the Ig genes. Patients with this specific rare form of leukemia (mature B-cell or Burkitt leukemia) should be treated according to protocols for Burkitt lymphoma. For more information about the treatment of mature B-cell lymphoma/leukemia and Burkitt lymphoma/leukemia, see Childhood Non-Hodgkin Lymphoma Treatment. Rarely, blasts with L1/L2 (not L3) morphology will express surface Ig.[
Risk Factors for Developing ALL
The primary accepted risk factors for ALL and associated genes (when relevant) include the following:
- Prenatal exposure to x-rays.
- Postnatal exposure to high doses of radiation (e.g., therapeutic radiation previously used for conditions such as tinea capitis and thymus enlargement).
- Previous treatment with chemotherapy.
- Genetic conditions that include the following:
- Down syndrome. For more information, see the Down syndrome section.
- Neurofibromatosis (NF1).[
14 ] - Bloom syndrome (BLM).[
15 ] - Fanconi anemia (multiple genes; ALL is observed much less frequently than acute myeloid leukemia [AML]).[
16 ] - Ataxia telangiectasia (ATM).[
17 ] - Li-Fraumeni syndrome (TP53).[
18 ,19 ,20 ] - Constitutional mismatch repair deficiency (biallelic variant of MLH1, MSH2, MSH6, and PMS2).[
21 ,22 ]
- Low- and high-penetrance inherited genetic variants.[
23 ] For more information, see the Low- and high-penetrance inherited genetic variants section. - Carriers of a constitutional Robertsonian translocation that involves chromosomes 15 and 21 and carriers of constitutional ring chromosome 21 are specifically and highly predisposed to developing intrachromosomal amplification of chromosome 21 (iAMP21) ALL.[
24 ,25 ]
Down syndrome
Children with Down syndrome have an increased risk of developing both ALL and AML,[
A genome-wide association study found that four susceptibility loci associated with B-ALL in the non-Down syndrome population (IKZF1, CDKN2A, ARID5B, and GATA3) were also associated with susceptibility to ALL in children with Down syndrome.[
Approximately one-half to two-thirds of cases of acute leukemia in children with Down syndrome are ALL, and about 2% to 3% of childhood ALL cases occur in children with Down syndrome.[
Patients with ALL and Down syndrome have a lower incidence of both favorable (ETV6::RUNX1 fusion and hyperdiploidy [51–65 chromosomes]) and unfavorable (BCR::ABL1 or KMT2A::AFF1 fusions and hypodiploidy [<44 chromosomes]) genomic alterations and a near absence of T-cell phenotype.[
Approximately 50% to 60% of cases of ALL in children with Down syndrome have genomic alterations affecting CRLF2 that generally result in overexpression of the protein produced by this gene, which dimerizes with the interleukin-7 receptor alpha to form the receptor for the cytokine thymic stromal lymphopoietin.[
Based on the relatively small number of published series, it does not appear that genomic CRLF2 aberrations in patients with Down syndrome and ALL have prognostic relevance.[
Approximately 20% to 30% of ALL cases arising in children with Down syndrome have somatically acquired JAK1 or JAK2 variants,[
IKZF1 gene deletions, observed in 20% to 35% of patients with Down syndrome and ALL, have been associated with a significantly worse outcome in this group of patients.[
Approximately 10% of patients with Down syndrome and ALL have genomic alterations leading to overexpression or abnormal activation of the CEBPD, CEBPA, and CEBPE genes.[
Low- and high-penetrance inherited genetic variants
Genetic predisposition to ALL can be divided into several broad categories, as follows:
- Association with genetic syndromes. Increased risk can be associated with the genetic syndromes listed above in which ALL is observed, although it is not the primary manifestation of the condition.
- Common alleles. Another category for genetic predisposition includes common alleles with relatively small effect sizes that are identified by genome-wide association studies. Genome-wide association studies have identified a number of germline (inherited) genetic polymorphisms that are associated with the development of childhood ALL.[
23 ] For example, the risk alleles of ARID5B are associated with the development of hyperdiploid (51–65 chromosomes) B-ALL. ARID5B is a gene that encodes a transcriptional factor important in embryonic development, cell type–specific gene expression, and cell growth regulation.[49 ,50 ] Other genes with polymorphisms associated with increased risk of ALL include GATA3,[51 ]IKZF1,[49 ,50 ,52 ]CDKN2A,[53 ]CDKN2B,[52 ,53 ]CEBPE,[49 ]PIP4K2A,[51 ,54 ] and TP63.[55 ]Genetic risk factors for T-ALL share some overlap with the genetic risk factors for B-ALL, but unique risk factors also exist. A genome-wide association study identified a risk allele near USP7 that was associated with an increased risk of developing T-ALL (odds ratio, 1.44) but not B-ALL. The risk allele was shown to be associated with reduced USP7 transcription, which is consistent with the finding that somatic loss-of-function variants in USP7 are observed in patients with T-ALL. USP7 germline and somatic variants are generally mutually exclusive and are most commonly observed in T-ALL patients with TAL1 alterations.[
56 ]Genetic risk factors that have similar impact for developing both B-ALL and T-ALL include CDKN2A, CDKN2B, and 8q24.21 (cis distal enhancer region variants for MYC).[
56 ] - Rare germline variants with high penetrance. Germline variants that cause pathogenic changes in genes associated with ALL and that are observed in kindreds with familial ALL (i.e., large effect sizes) comprise another category of genetic predisposition to ALL. Many of the genes associated with ALL risk play key roles in B-cell development (e.g., PAX5, ETV6, and IKZF1).[
57 ]- PAX5. A germline variant in PAX5 that substitutes serine for glycine at amino acid 183 and that reduces PAX5 activity has been identified in several families that experienced multiple cases of ALL.[
58 ,59 ] - ETV6. Several germline ETV6 variants that lead to loss of ETV6 function have been identified in kindreds affected by both thrombocytopenia and ALL.[
60 ,61 ,62 ,63 ,64 ] Sequencing of ETV6 in remission (i.e., germline) specimens identified variants that were potentially related to ALL in approximately 1% of children with ALL that were evaluated.[60 ] Most of the germline variants (approximately 75%) were shown to be deleterious for ETV6 function, and 70% of cases with a deleterious germline ETV6 variant had a hyperdiploid karyotype. The remaining cases with a deleterious variant had diploid ALL, with a transcriptional profile similar to that of cases with ETV6::RUNX1 fusion–positive ALL.[64 ] - TP53. Pathogenic germline TP53 variants are associated with an increased risk of ALL.[
65 ] A study of 3,801 children with ALL observed that 26 patients (0.7%) had a pathogenic TP53 germline variant, with an associated odds ratio of 5.2 for ALL development.[65 ] Compared with ALL in children with TP53 wild-type status or TP53 variants of unknown significance, ALL in children with pathogenic germline TP53 variants was associated with older age at diagnosis (15.5 years vs. 7.3 years), hypodiploidy (65% vs. 1%), inferior EFS and overall survival, and a higher risk of second cancers. - IKZF1. Germline IKZF1 variants were identified in a kindred with familial ALL and in 43 of 4,963 (0.9%) children with sporadic ALL. Most (22 of 28) IKZF1 variants were shown to adversely affect IKZF1 gene function.[
66 ] Germline variants in IKZF1 have been identified in hereditary hypogammaglobulinemia. In one series, 2 of 29 affected patients developed B-ALL during childhood.[67 ]
- PAX5. A germline variant in PAX5 that substitutes serine for glycine at amino acid 183 and that reduces PAX5 activity has been identified in several families that experienced multiple cases of ALL.[
Prenatal origin of childhood ALL
Development of ALL is a multistep process in most cases, with more than one genomic alteration required for frank leukemia to develop. In at least some cases of childhood ALL, the initial genomic alteration occurs in utero. Evidence to support this comes from the observation that the immunoglobulin or T-cell receptor antigen rearrangements that are unique to each patient's leukemia cells can be detected in blood samples obtained at birth.[
Evidence also exists that some children who never develop ALL are born with rare blood cells carrying a genomic alteration associated with ALL. Initial studies focused on the ETV6::RUNX1 translocation and used reverse transcriptase–polymerase chain reaction (PCR) to identify RNA transcripts indicating the presence of the gene fusion. For example, in one study, 1% of neonatal blood spots (Guthrie cards) tested positive for the ETV6::RUNX1 translocation.[
To more definitively address this question, a highly sensitive and specific DNA-based approach (genomic inverse PCR for exploration of ligated breakpoints) was applied to DNA from 1,000 cord blood specimens and found that 5% of specimens had the ETV6::RUNX1 translocation.[
Clinical Presentation
The typical and atypical symptoms and clinical findings of childhood ALL have been published.[
Diagnosis
The evaluation needed to definitively diagnose childhood ALL has been published.[
Overall Prognosis
Among children with ALL, approximately 98% attain remission. Approximately 85% of patients aged 1 to 18 years with newly diagnosed ALL treated on current regimens are expected to be long-term event-free survivors, with more than 90% of patients alive at 5 years.[
Cytogenetic and genomic findings combined with minimal residual disease (MRD) results can define subsets of ALL with EFS rates exceeding 95% and, conversely, subsets with EFS rates of 50% or lower. For more information, see the sections on Cytogenetics/Genomics of Childhood ALL and Prognostic Factors Affecting Risk-Based Treatment.
Despite the treatment advances in childhood ALL, numerous important biological and therapeutic questions remain to be answered before the goal of curing every child with ALL with the least associated toxicity can be achieved. The systematic investigation of these issues requires large clinical trials, and the opportunity to participate in these trials is offered to most patients and families.
Clinical trials for children and adolescents with ALL are generally designed to compare therapy that is currently accepted as standard with investigational regimens that seek to improve cure rates and/or decrease toxicity. In certain trials in which the cure rate for the patient group is very high, therapy reduction questions may be asked. Much of the progress made in identifying curative therapies for childhood ALL and other childhood cancers has been achieved through investigator-driven discovery and tested in carefully randomized, controlled, multi-institutional clinical trials. Information about ongoing clinical trials is available from the
Current Clinical Trials
Use our
References:
- Smith MA, Altekruse SF, Adamson PC, et al.: Declining childhood and adolescent cancer mortality. Cancer 120 (16): 2497-506, 2014.
- Surveillance Research Program, National Cancer Institute: SEER*Explorer: An interactive website for SEER cancer statistics. Bethesda, MD: National Cancer Institute.
Available online . Last accessed March 6, 2024. - National Cancer Institute: NCCR*Explorer: An interactive website for NCCR cancer statistics. Bethesda, MD: National Cancer Institute.
Available online . Last accessed August 23, 2024. - Childhood cancer. In: Howlader N, Noone AM, Krapcho M, et al., eds.: SEER Cancer Statistics Review, 1975-2010. National Cancer Institute, 2013, Section 28.
Also available online . Last accessed August 21, 2023. - Childhood cancer by the ICCC. In: Howlader N, Noone AM, Krapcho M, et al., eds.: SEER Cancer Statistics Review, 1975-2010. National Cancer Institute, 2013, Section 29.
Also available online . Last accessed August 21, 2023. - Howlader N, Noone AM, Krapcho M: SEER Cancer Statistics Review (CSR) 1975-2013. Bethesda, Md: National Cancer Institute, 2015.
Available online . Last accessed June 04, 2021. - Surveillance, Epidemiology, and End Results Program: SEER Cancer Stat Facts: Childhood Leukemia (Ages 0–19). Bethesda, Md: National Cancer Institute, DCCPS, Surveillance Research Program.
Available online . Last accessed September 7, 2022. - Special section: cancer in children and adolescents. In: American Cancer Society: Cancer Facts and Figures 2014. American Cancer Society, 2014, pp 25-42.
Available online . Last accessed June 04, 2021. - Shah A, Coleman MP: Increasing incidence of childhood leukaemia: a controversy re-examined. Br J Cancer 97 (7): 1009-12, 2007.
- Smith MA, Ries LA, Gurney JG, et al.: Leukemia. In: Ries LA, Smith MA, Gurney JG, et al., eds.: Cancer incidence and survival among children and adolescents: United States SEER Program 1975-1995. National Cancer Institute, SEER Program, 1999. NIH Pub.No. 99-4649, pp 17-34.
Also available online . Last accessed August 11, 2022. - Barrington-Trimis JL, Cockburn M, Metayer C, et al.: Rising rates of acute lymphoblastic leukemia in Hispanic children: trends in incidence from 1992 to 2011. Blood 125 (19): 3033-4, 2015.
- Bennett JM, Catovsky D, Daniel MT, et al.: The morphological classification of acute lymphoblastic leukaemia: concordance among observers and clinical correlations. Br J Haematol 47 (4): 553-61, 1981.
- Koehler M, Behm FG, Shuster J, et al.: Transitional pre-B-cell acute lymphoblastic leukemia of childhood is associated with favorable prognostic clinical features and an excellent outcome: a Pediatric Oncology Group study. Leukemia 7 (12): 2064-8, 1993.
- Stiller CA, Chessells JM, Fitchett M: Neurofibromatosis and childhood leukaemia/lymphoma: a population-based UKCCSG study. Br J Cancer 70 (5): 969-72, 1994.
- Passarge E: Bloom's syndrome: the German experience. Ann Genet 34 (3-4): 179-97, 1991.
- Alter BP: Cancer in Fanconi anemia, 1927-2001. Cancer 97 (2): 425-40, 2003.
- Taylor AM, Metcalfe JA, Thick J, et al.: Leukemia and lymphoma in ataxia telangiectasia. Blood 87 (2): 423-38, 1996.
- Holmfeldt L, Wei L, Diaz-Flores E, et al.: The genomic landscape of hypodiploid acute lymphoblastic leukemia. Nat Genet 45 (3): 242-52, 2013.
- Powell BC, Jiang L, Muzny DM, et al.: Identification of TP53 as an acute lymphocytic leukemia susceptibility gene through exome sequencing. Pediatr Blood Cancer 60 (6): E1-3, 2013.
- Hof J, Krentz S, van Schewick C, et al.: Mutations and deletions of the TP53 gene predict nonresponse to treatment and poor outcome in first relapse of childhood acute lymphoblastic leukemia. J Clin Oncol 29 (23): 3185-93, 2011.
- Ilencikova D, Sejnova D, Jindrova J, et al.: High-grade brain tumors in siblings with biallelic MSH6 mutations. Pediatr Blood Cancer 57 (6): 1067-70, 2011.
- Ripperger T, Schlegelberger B: Acute lymphoblastic leukemia and lymphoma in the context of constitutional mismatch repair deficiency syndrome. Eur J Med Genet 59 (3): 133-42, 2016.
- Moriyama T, Relling MV, Yang JJ: Inherited genetic variation in childhood acute lymphoblastic leukemia. Blood 125 (26): 3988-95, 2015.
- Li Y, Schwab C, Ryan SL, et al.: Constitutional and somatic rearrangement of chromosome 21 in acute lymphoblastic leukaemia. Nature 508 (7494): 98-102, 2014.
- Harrison CJ, Moorman AV, Schwab C, et al.: An international study of intrachromosomal amplification of chromosome 21 (iAMP21): cytogenetic characterization and outcome. Leukemia 28 (5): 1015-21, 2014.
- Hasle H: Pattern of malignant disorders in individuals with Down's syndrome. Lancet Oncol 2 (7): 429-36, 2001.
- Lupo PJ, Schraw JM, Desrosiers TA, et al.: Association Between Birth Defects and Cancer Risk Among Children and Adolescents in a Population-Based Assessment of 10 Million Live Births. JAMA Oncol 5 (8): 1150-1158, 2019.
- Marlow EC, Ducore J, Kwan ML, et al.: Leukemia Risk in a Cohort of 3.9 Million Children with and without Down Syndrome. J Pediatr 234: 172-180.e3, 2021.
- Brown AL, de Smith AJ, Gant VU, et al.: Inherited genetic susceptibility to acute lymphoblastic leukemia in Down syndrome. Blood 134 (15): 1227-1237, 2019.
- Zeller B, Gustafsson G, Forestier E, et al.: Acute leukaemia in children with Down syndrome: a population-based Nordic study. Br J Haematol 128 (6): 797-804, 2005.
- Arico M, Ziino O, Valsecchi MG, et al.: Acute lymphoblastic leukemia and Down syndrome: presenting features and treatment outcome in the experience of the Italian Association of Pediatric Hematology and Oncology (AIEOP). Cancer 113 (3): 515-21, 2008.
- Maloney KW, Carroll WL, Carroll AJ, et al.: Down syndrome childhood acute lymphoblastic leukemia has a unique spectrum of sentinel cytogenetic lesions that influences treatment outcome: a report from the Children's Oncology Group. Blood 116 (7): 1045-50, 2010.
- de Graaf G, Buckley F, Skotko BG: Estimation of the number of people with Down syndrome in the United States. Genet Med 19 (4): 439-447, 2017.
- Chessells JM, Harrison G, Richards SM, et al.: Down's syndrome and acute lymphoblastic leukaemia: clinical features and response to treatment. Arch Dis Child 85 (4): 321-5, 2001.
- Buitenkamp TD, Izraeli S, Zimmermann M, et al.: Acute lymphoblastic leukemia in children with Down syndrome: a retrospective analysis from the Ponte di Legno study group. Blood 123 (1): 70-7, 2014.
- Hertzberg L, Vendramini E, Ganmore I, et al.: Down syndrome acute lymphoblastic leukemia, a highly heterogeneous disease in which aberrant expression of CRLF2 is associated with mutated JAK2: a report from the International BFM Study Group. Blood 115 (5): 1006-17, 2010.
- Buitenkamp TD, Pieters R, Gallimore NE, et al.: Outcome in children with Down's syndrome and acute lymphoblastic leukemia: role of IKZF1 deletions and CRLF2 aberrations. Leukemia 26 (10): 2204-11, 2012.
- Mullighan CG, Collins-Underwood JR, Phillips LA, et al.: Rearrangement of CRLF2 in B-progenitor- and Down syndrome-associated acute lymphoblastic leukemia. Nat Genet 41 (11): 1243-6, 2009.
- Russell LJ, Jones L, Enshaei A, et al.: Characterisation of the genomic landscape of CRLF2-rearranged acute lymphoblastic leukemia. Genes Chromosomes Cancer 56 (5): 363-372, 2017.
- Harvey RC, Mullighan CG, Chen IM, et al.: Rearrangement of CRLF2 is associated with mutation of JAK kinases, alteration of IKZF1, Hispanic/Latino ethnicity, and a poor outcome in pediatric B-progenitor acute lymphoblastic leukemia. Blood 115 (26): 5312-21, 2010.
- Schwab CJ, Chilton L, Morrison H, et al.: Genes commonly deleted in childhood B-cell precursor acute lymphoblastic leukemia: association with cytogenetics and clinical features. Haematologica 98 (7): 1081-8, 2013.
- Li Z, Chang TC, Junco JJ, et al.: Genomic landscape of Down syndrome-associated acute lymphoblastic leukemia. Blood 142 (2): 172-184, 2023.
- Bercovich D, Ganmore I, Scott LM, et al.: Mutations of JAK2 in acute lymphoblastic leukaemias associated with Down's syndrome. Lancet 372 (9648): 1484-92, 2008.
- Gaikwad A, Rye CL, Devidas M, et al.: Prevalence and clinical correlates of JAK2 mutations in Down syndrome acute lymphoblastic leukaemia. Br J Haematol 144 (6): 930-2, 2009.
- Kearney L, Gonzalez De Castro D, Yeung J, et al.: Specific JAK2 mutation (JAK2R683) and multiple gene deletions in Down syndrome acute lymphoblastic leukemia. Blood 113 (3): 646-8, 2009.
- Mullighan CG, Zhang J, Harvey RC, et al.: JAK mutations in high-risk childhood acute lymphoblastic leukemia. Proc Natl Acad Sci U S A 106 (23): 9414-8, 2009.
- Hanada I, Terui K, Ikeda F, et al.: Gene alterations involving the CRLF2-JAK pathway and recurrent gene deletions in Down syndrome-associated acute lymphoblastic leukemia in Japan. Genes Chromosomes Cancer 53 (11): 902-10, 2014.
- Michels N, Boer JM, Enshaei A, et al.: Minimal residual disease, long-term outcome, and IKZF1 deletions in children and adolescents with Down syndrome and acute lymphocytic leukaemia: a matched cohort study. Lancet Haematol 8 (10): e700-e710, 2021.
- Papaemmanuil E, Hosking FJ, Vijayakrishnan J, et al.: Loci on 7p12.2, 10q21.2 and 14q11.2 are associated with risk of childhood acute lymphoblastic leukemia. Nat Genet 41 (9): 1006-10, 2009.
- Treviño LR, Yang W, French D, et al.: Germline genomic variants associated with childhood acute lymphoblastic leukemia. Nat Genet 41 (9): 1001-5, 2009.
- Migliorini G, Fiege B, Hosking FJ, et al.: Variation at 10p12.2 and 10p14 influences risk of childhood B-cell acute lymphoblastic leukemia and phenotype. Blood 122 (19): 3298-307, 2013.
- Hungate EA, Vora SR, Gamazon ER, et al.: A variant at 9p21.3 functionally implicates CDKN2B in paediatric B-cell precursor acute lymphoblastic leukaemia aetiology. Nat Commun 7: 10635, 2016.
- Sherborne AL, Hosking FJ, Prasad RB, et al.: Variation in CDKN2A at 9p21.3 influences childhood acute lymphoblastic leukemia risk. Nat Genet 42 (6): 492-4, 2010.
- Xu H, Yang W, Perez-Andreu V, et al.: Novel susceptibility variants at 10p12.31-12.2 for childhood acute lymphoblastic leukemia in ethnically diverse populations. J Natl Cancer Inst 105 (10): 733-42, 2013.
- Ellinghaus E, Stanulla M, Richter G, et al.: Identification of germline susceptibility loci in ETV6-RUNX1-rearranged childhood acute lymphoblastic leukemia. Leukemia 26 (5): 902-9, 2012.
- Qian M, Zhao X, Devidas M, et al.: Genome-Wide Association Study of Susceptibility Loci for T-Cell Acute Lymphoblastic Leukemia in Children. J Natl Cancer Inst 111 (12): 1350-1357, 2019.
- Somasundaram R, Prasad MA, Ungerbäck J, et al.: Transcription factor networks in B-cell differentiation link development to acute lymphoid leukemia. Blood 126 (2): 144-52, 2015.
- Shah S, Schrader KA, Waanders E, et al.: A recurrent germline PAX5 mutation confers susceptibility to pre-B cell acute lymphoblastic leukemia. Nat Genet 45 (10): 1226-31, 2013.
- Auer F, Rüschendorf F, Gombert M, et al.: Inherited susceptibility to pre B-ALL caused by germline transmission of PAX5 c.547G>A. Leukemia 28 (5): 1136-8, 2014.
- Zhang MY, Churpek JE, Keel SB, et al.: Germline ETV6 mutations in familial thrombocytopenia and hematologic malignancy. Nat Genet 47 (2): 180-5, 2015.
- Topka S, Vijai J, Walsh MF, et al.: Germline ETV6 Mutations Confer Susceptibility to Acute Lymphoblastic Leukemia and Thrombocytopenia. PLoS Genet 11 (6): e1005262, 2015.
- Noetzli L, Lo RW, Lee-Sherick AB, et al.: Germline mutations in ETV6 are associated with thrombocytopenia, red cell macrocytosis and predisposition to lymphoblastic leukemia. Nat Genet 47 (5): 535-8, 2015.
- Rampersaud E, Ziegler DS, Iacobucci I, et al.: Germline deletion of ETV6 in familial acute lymphoblastic leukemia. Blood Adv 3 (7): 1039-1046, 2019.
- Nishii R, Baskin-Doerfler R, Yang W, et al.: Molecular basis of ETV6-mediated predisposition to childhood acute lymphoblastic leukemia. Blood 137 (3): 364-373, 2021.
- Qian M, Cao X, Devidas M, et al.: TP53 Germline Variations Influence the Predisposition and Prognosis of B-Cell Acute Lymphoblastic Leukemia in Children. J Clin Oncol 36 (6): 591-599, 2018.
- Churchman ML, Qian M, Te Kronnie G, et al.: Germline Genetic IKZF1 Variation and Predisposition to Childhood Acute Lymphoblastic Leukemia. Cancer Cell 33 (5): 937-948.e8, 2018.
- Kuehn HS, Boisson B, Cunningham-Rundles C, et al.: Loss of B Cells in Patients with Heterozygous Mutations in IKAROS. N Engl J Med 374 (11): 1032-1043, 2016.
- Greaves MF, Wiemels J: Origins of chromosome translocations in childhood leukaemia. Nat Rev Cancer 3 (9): 639-49, 2003.
- Taub JW, Konrad MA, Ge Y, et al.: High frequency of leukemic clones in newborn screening blood samples of children with B-precursor acute lymphoblastic leukemia. Blood 99 (8): 2992-6, 2002.
- Bateman CM, Colman SM, Chaplin T, et al.: Acquisition of genome-wide copy number alterations in monozygotic twins with acute lymphoblastic leukemia. Blood 115 (17): 3553-8, 2010.
- Greaves MF, Maia AT, Wiemels JL, et al.: Leukemia in twins: lessons in natural history. Blood 102 (7): 2321-33, 2003.
- Mori H, Colman SM, Xiao Z, et al.: Chromosome translocations and covert leukemic clones are generated during normal fetal development. Proc Natl Acad Sci U S A 99 (12): 8242-7, 2002.
- Schäfer D, Olsen M, Lähnemann D, et al.: Five percent of healthy newborns have an ETV6-RUNX1 fusion as revealed by DNA-based GIPFEL screening. Blood 131 (7): 821-826, 2018.
- Hein D, Dreisig K, Metzler M, et al.: The preleukemic TCF3-PBX1 gene fusion can be generated in utero and is present in ≈0.6% of healthy newborns. Blood 134 (16): 1355-1358, 2019.
- Gramatges MM, O'Brien MM, Rabin KR: Acute lymphoblastic leukemia. In: Blaney SM, Helman LJ, Adamson PC, eds.: Pizzo and Poplack's Pediatric Oncology. 8th ed. Wolters Kluwer, 2020, pp 419-53.
- Chessells JM; haemostasis and thrombosis task force, British committee for standards in haematology: Pitfalls in the diagnosis of childhood leukaemia. Br J Haematol 114 (3): 506-11, 2001.
- Onciu M: Acute lymphoblastic leukemia. Hematol Oncol Clin North Am 23 (4): 655-74, 2009.
- Margolskee E, Waith Wertheim GB, Harvey RC: Pathology and molecular diagnosis of leukemias and lymphomas. In: Blaney SM, Helman LJ, Adamson PC, eds.: Pizzo and Poplack's Pediatric Oncology. 8th ed. Wolters Kluwer, 2020, pp 117-30.
- Cheng J, Klairmont MM, Choi JK: Peripheral blood flow cytometry for the diagnosis of pediatric acute leukemia: Highly reliable with rare exceptions. Pediatr Blood Cancer 66 (1): e27453, 2019.
- Möricke A, Zimmermann M, Valsecchi MG, et al.: Dexamethasone vs prednisone in induction treatment of pediatric ALL: results of the randomized trial AIEOP-BFM ALL 2000. Blood 127 (17): 2101-12, 2016.
- Vora A, Goulden N, Wade R, et al.: Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): a randomised controlled trial. Lancet Oncol 14 (3): 199-209, 2013.
- Place AE, Stevenson KE, Vrooman LM, et al.: Intravenous pegylated asparaginase versus intramuscular native Escherichia coli L-asparaginase in newly diagnosed childhood acute lymphoblastic leukaemia (DFCI 05-001): a randomised, open-label phase 3 trial. Lancet Oncol 16 (16): 1677-90, 2015.
- Pieters R, de Groot-Kruseman H, Van der Velden V, et al.: Successful Therapy Reduction and Intensification for Childhood Acute Lymphoblastic Leukemia Based on Minimal Residual Disease Monitoring: Study ALL10 From the Dutch Childhood Oncology Group. J Clin Oncol 34 (22): 2591-601, 2016.
- Moorman AV, Antony G, Wade R, et al.: Time to Cure for Childhood and Young Adult Acute Lymphoblastic Leukemia Is Independent of Early Risk Factors: Long-Term Follow-Up of the UKALL2003 Trial. J Clin Oncol 40 (36): 4228-4239, 2022.
World Health Organization (WHO) Classification System for Childhood ALL
The 5th edition of the WHO Classification of Haematolymphoid Tumours lists the following entities for acute lymphoid leukemias:[
WHO 5th Edition Classification of B-Cell Lymphoblastic Leukemias/Lymphomas
- B-lymphoblastic leukemia/lymphoma, not otherwise specified (NOS).
- B-lymphoblastic leukemia/lymphoma with high hyperdiploidy.
- B-lymphoblastic leukemia/lymphoma with iAMP21.
- B-lymphoblastic leukemia/lymphoma with BCR::ABL1 fusion.
- B-lymphoblastic leukemia/lymphoma with BCR::ABL1-like features.
- B-lymphoblastic leukemia/lymphoma with KMT2A rearrangement.
- B-lymphoblastic leukemia/lymphoma with ETV6::RUNX1 fusion.
- B-lymphoblastic leukemia/lymphoma with ETV6::RUNX1-like features.
- B-lymphoblastic leukemia/lymphoma with TCF3::PBX1 fusion.
- B-lymphoblastic leukemia/lymphoma with IGH::IL3 fusion.
- B-lymphoblastic leukemia/lymphoma with TCF3::HLF fusion.
- B-lymphoblastic leukemia/lymphoma with other defined genetic abnormalities.
The category of B-ALL with other defined genetic abnormalities includes potential novel entities, including B-ALL with DUX4, MEF2D, ZNF384 or NUTM1 rearrangements; B-ALL with IG::MYC fusions; and B-ALL with PAX5alt or PAX5 p.P80R (NP_057953.1) abnormalities.
WHO 5th Edition Classification of T-Lymphoblastic Leukemia/Lymphoma
- T-lymphoblastic leukemia/lymphoma, NOS.
- Early T-precursor lymphoblastic leukemia/lymphoma.
2016 WHO Classification of Acute Leukemias of Ambiguous Lineage
For acute leukemias of ambiguous lineage, the group of acute leukemias that have characteristics of both acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL), the WHO classification system is summarized in Table 1.[
| Condition | Definition |
|---|---|
| MPAL = mixed phenotype acute leukemia; NOS = not otherwise specified. | |
| a Adapted from Béné MC: Biphenotypic, bilineal, ambiguous or mixed lineage: strange leukemias! Haematologica 94 (7): 891-3, 2009.[ |
|
| Acute undifferentiated leukemia | Acute leukemia that does not express any marker considered specific for either lymphoid or myeloid lineage |
| MPAL withBCR::ABL1(t(9;22)(q34;q11.2)) | Acute leukemia meeting the diagnostic criteria for mixed phenotype acute leukemia in which the blasts also have the (9;22) translocation or theBCR::ABL1rearrangement |
| MPAL withKMT2Arearranged (t(v;11q23)) | Acute leukemia meeting the diagnostic criteria for mixed phenotype acute leukemia in which the blasts also have a translocation involving theKMT2Agene |
| MPAL, B/myeloid, NOS (B/M MPAL) | Acute leukemia meeting the diagnostic criteria for assignment to both B and myeloid lineage, in which the blasts lack genetic abnormalities involvingBCR::ABL1orKMT2A |
| MPAL, T/myeloid, NOS (T/M MPAL) | Acute leukemia meeting the diagnostic criteria for assignment to both T and myeloid lineage, in which the blasts lack genetic abnormalities involvingBCR::ABL1orKMT2A |
| MPAL, B/myeloid, NOS—rare types | Acute leukemia meeting the diagnostic criteria for assignment to both B and T lineage |
| Other ambiguous lineage leukemias | Natural killer–cell lymphoblastic leukemia/lymphoma |
| Lineage | Criteria |
|---|---|
| a Adapted from Arber et al.[ |
|
| b Strong defined as equal to or brighter than the normal B or T cells in the sample. | |
| Myeloid lineage | Myeloperoxidase (flow cytometry, immunohistochemistry, or cytochemistry);or monocytic differentiation (at least two of the following: nonspecific esterase cytochemistry, CD11c, CD14, CD64, lysozyme) |
| T lineage | Strongb cytoplasmic CD3 (with antibodies to CD3 epsilon chain);or surface CD3 |
| B lineage | Strongb CD19 with at least one of the following strongly expressed: CD79a, cytoplasmic CD22, or CD10;or weak CD19 with at least two of the following strongly expressed: CD79a, cytoplasmic CD22, or CD10 |
Leukemias of mixed phenotype may be seen in various presentations, including the following:
- Bilineal leukemias in which there are two distinct populations of cells, usually one lymphoid and one myeloid.
- Biphenotypic leukemias in which individual blast cells display features of both lymphoid and myeloid lineage.
Biphenotypic cases represent most of the mixed phenotype leukemias.[
Some studies suggest that patients with biphenotypic leukemia may fare better with a lymphoid, as opposed to a myeloid, treatment regimen.[
For more information about key clinical and biological characteristics, as well as the prognostic significance for these entities, see the Cytogenetics/Genomics of Childhood ALL section.
References:
- Alaggio R, Amador C, Anagnostopoulos I, et al.: The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 36 (7): 1720-1748, 2022.
- Béné MC: Biphenotypic, bilineal, ambiguous or mixed lineage: strange leukemias! Haematologica 94 (7): 891-3, 2009.
- Borowitz MJ, Béné MC, Harris NL: Acute leukaemias of ambiguous lineage. In: Swerdlow SH, Campo E, Harris NL, et al., eds.: WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. International Agency for Research on Cancer, 2008, pp 150-5.
- Arber DA, Orazi A, Hasserjian R, et al.: The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127 (20): 2391-405, 2016.
- Gerr H, Zimmermann M, Schrappe M, et al.: Acute leukaemias of ambiguous lineage in children: characterization, prognosis and therapy recommendations. Br J Haematol 149 (1): 84-92, 2010.
- Shago M, Abla O, Hitzler J, et al.: Frequency and outcome of pediatric acute lymphoblastic leukemia with ZNF384 gene rearrangements including a novel translocation resulting in an ARID1B/ZNF384 gene fusion. Pediatr Blood Cancer 63 (11): 1915-21, 2016.
- Yao L, Cen J, Pan J, et al.: TAF15-ZNF384 fusion gene in childhood mixed phenotype acute leukemia. Cancer Genet 211: 1-4, 2017.
- Alexander TB, Gu Z, Iacobucci I, et al.: The genetic basis and cell of origin of mixed phenotype acute leukaemia. Nature 562 (7727): 373-379, 2018.
- Rubnitz JE, Onciu M, Pounds S, et al.: Acute mixed lineage leukemia in children: the experience of St Jude Children's Research Hospital. Blood 113 (21): 5083-9, 2009.
- Al-Seraihy AS, Owaidah TM, Ayas M, et al.: Clinical characteristics and outcome of children with biphenotypic acute leukemia. Haematologica 94 (12): 1682-90, 2009.
- Matutes E, Pickl WF, Van't Veer M, et al.: Mixed-phenotype acute leukemia: clinical and laboratory features and outcome in 100 patients defined according to the WHO 2008 classification. Blood 117 (11): 3163-71, 2011.
- Hrusak O, de Haas V, Stancikova J, et al.: International cooperative study identifies treatment strategy in childhood ambiguous lineage leukemia. Blood 132 (3): 264-276, 2018.
- Orgel E, Alexander TB, Wood BL, et al.: Mixed-phenotype acute leukemia: A cohort and consensus research strategy from the Children's Oncology Group Acute Leukemia of Ambiguous Lineage Task Force. Cancer 126 (3): 593-601, 2020.
Cytogenetics / Genomics of Childhood ALL
Genomics of childhood ALL
The genomics of childhood acute lymphoblastic leukemia (ALL) has been extensively investigated, and multiple distinctive subtypes have been defined on the basis of cytogenetic and molecular characterizations, each with its own pattern of clinical and prognostic characteristics.[
Throughout this section, the percentages of genomic subtypes from among all B-ALL and T-ALL cases are derived primarily from a report describing the genomic characterization of patients treated on several Children's Oncology Group (COG) and St. Jude Children's Research Hospital (SJCRH) clinical trials. Percentages by subtype are presented for NCI standard-risk and NCI high-risk patients with B-ALL (up to age 18 years).[
B-ALL cytogenetics/genomics
B-ALL is typified by genomic alterations that include: 1) gene fusions that lead to aberrant activity of transcription factors, 2) chromosomal gains and losses (e.g., hyperdiploidy or hypodiploidy), and 3) alterations leading to activation of tyrosine kinase genes.[
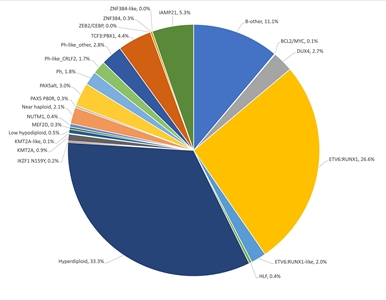
Figure 2. Genomic subtypes and frequencies of NCI standard-risk B-ALL. The figure represents data from 1,126 children diagnosed with NCI standard-risk B-ALL (aged 1–9 years and WBC <50,000/µL) and enrolled in St. Jude Children's Research Hospital or Children's Oncology Group clinical trials. Adapted from Supplemental Table 2 of Brady SW, Roberts KG, Gu Z, et al.: The genomic landscape of pediatric acute lymphoblastic leukemia. Nature Genetics 54: 1376-1389, 2022.
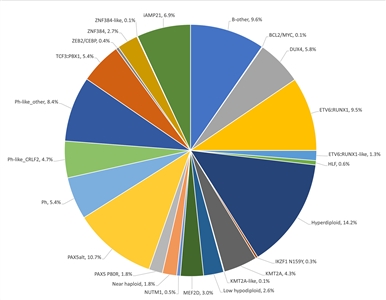
Figure 3. Genomic subtypes and frequencies of NCI high-risk B-ALL. The figure represents data from 1,084 children diagnosed with NCI high-risk B-ALL (aged 1–18 years and WBC >50,000/µL) and enrolled in St. Jude Children's Research Hospital or Children's Oncology Group clinical trials. Adapted from Supplemental Table 2 of Brady SW, Roberts KG, Gu Z, et al.: The genomic landscape of pediatric acute lymphoblastic leukemia. Nature Genetics 54: 1376-1389, 2022.
The genomic landscape of B-ALL is characterized by a range of genomic alterations that disrupt normal B-cell development and, in some cases, by variants in genes that provide a proliferation signal (e.g., activating variants in RAS family genes or variants/translocations leading to kinase pathway signaling). Genomic alterations leading to blockage of B-cell development include translocations (e.g., TCF3::PBX1 and ETV6::RUNX1 fusions), point variants (e.g., IKZF1 and PAX5), and intragenic/intergenic deletions (e.g., IKZF1, PAX5, EBF, and ERG).[
The genomic alterations in B-ALL tend not to occur at random, but rather to cluster within subtypes that can be delineated by biological characteristics such as their gene expression profiles. Cases with recurring chromosomal translocations (e.g., TCF3::PBX1 and ETV6::RUNX1 fusions and KMT2A-rearranged ALL) have distinctive biological features and illustrate this point, as do the examples below of specific genomic alterations within unique biological subtypes:
- IKZF1 deletions and variants are most commonly observed within cases of BCR::ABL1 ALL and BCR::ABL1-like ALL.[
3 ,4 ] - Intragenic ERG deletions occur within a distinctive subtype characterized by gene rearrangements involving DUX4.[
5 ,6 ] - TP53 variants, often germline, occur at high frequency in patients with low hypodiploid ALL with 32 to 39 chromosomes.[
7 ]TP53 variants are uncommon in other patients with B-ALL.
Activating point variants in kinase genes are uncommon in high-risk B-ALL. JAK genes are the primary kinases that are found to be altered. These variants are generally observed in patients with BCR::ABL1-like ALL who have CRLF2 abnormalities, although JAK2 variants are also observed in approximately 25% of children with Down syndrome and ALL, occurring exclusively in cases with CRLF2 gene rearrangements.[
Understanding of the genomics of B-ALL at relapse is less advanced than the understanding of ALL genomics at diagnosis. Childhood ALL is often polyclonal at diagnosis and under the selective influence of therapy, some clones may be extinguished and new clones with distinctive genomic profiles may arise.[
Several recurrent chromosomal abnormalities have been shown to have prognostic significance, especially in B-ALL. Some chromosomal alterations are associated with more favorable outcomes, such as favorable trisomies (51–65 chromosomes) and the ETV6::RUNX1 fusion.[
In recognition of the clinical significance of many of these genomic alterations, the 5th edition revision of the World Health Organization Classification of Haematolymphoid Tumours lists the following entities for B-ALL:[
- B-lymphoblastic leukemia/lymphoma, NOS.
- B-lymphoblastic leukemia/lymphoma with high hyperdiploidy.
- B-lymphoblastic leukemia/lymphoma with hypodiploidy.
- B-lymphoblastic leukemia/lymphoma with iAMP21.
- B-lymphoblastic leukemia/lymphoma with BCR::ABL1 fusion.
- B-lymphoblastic leukemia/lymphoma with BCR::ABL1-like features.
- B-lymphoblastic leukemia/lymphoma with KMT2A rearrangement.
- B-lymphoblastic leukemia/lymphoma with ETV6::RUNX1 fusion.
- B-lymphoblastic leukemia/lymphoma with ETV6::RUNX1-like features.
- B-lymphoblastic leukemia/lymphoma with TCF3::PBX1 fusion.
- B-lymphoblastic leukemia/lymphoma with IGH::IL3 fusion.
- B-lymphoblastic leukemia/lymphoma with TCF3::HLF fusion.
- B-lymphoblastic leukemia/lymphoma with other defined genetic abnormalities.
The category of B-ALL with other defined genetic abnormalities includes potential novel entities, including B-ALL with DUX4, MEF2D, ZNF384 or NUTM1 rearrangements; B-ALL with IG::MYC fusions; and B-ALL with PAX5alt or PAX5 p.P80R (NP_057953.1) abnormalities.
These and other chromosomal and genomic abnormalities for childhood ALL are described below.
- Chromosome number.
- High hyperdiploidy (51–65 chromosomes).
High hyperdiploidy, defined as 51 to 65 chromosomes per cell or a DNA index greater than 1.16, occurs in approximately 33% of NCI standard-risk and 14% of NCI high-risk pediatric B-ALL cases.[
1 ,19 ] Hyperdiploidy can be evaluated by measuring the DNA content of cells (DNA index) or by karyotyping. In cases with a normal karyotype or in which standard cytogenetic analysis was unsuccessful, interphase fluorescence in situ hybridization (FISH) may detect hidden hyperdiploidy.High hyperdiploidy generally occurs in cases with clinically favorable prognostic factors (patients aged 1 to <10 years with a low white blood cell [WBC] count) and is an independent favorable prognostic factor.[
19 ,20 ,21 ] Within the hyperdiploid range of 51 to 65 chromosomes, patients with higher modal numbers (58–66) appeared to have a better prognosis in one study.[21 ] Hyperdiploid leukemia cells are particularly susceptible to undergoing apoptosis and accumulate higher levels of methotrexate and its active polyglutamate metabolites,[22 ] which may explain the favorable outcome commonly observed in these cases.While the overall outcome of patients with high hyperdiploidy is considered to be favorable, factors such as age, WBC count, specific trisomies, and early response to treatment have been shown to modify its prognostic significance.[
23 ,24 ]Multiple reports have described the prognostic significance of specific chromosome trisomies among children with hyperdiploid B-ALL.
- A study combining experience from the Children's Cancer Group and the Pediatric Oncology Group (POG) found that patients with trisomies of chromosomes 4, 10, and 17 (triple trisomies) have a particularly favorable outcome.[
25 ]; [16 ][Level of evidence B4] - A report using POG data found that NCI standard-risk patients with trisomies of 4 and 10, without regard to chromosome 17 status, have an excellent prognosis.[
26 ] COG protocols currently use double trisomies of chromosomes 4 and 10 to define favorable hyperdiploidy. - A retrospective analysis evaluated patients treated on two consecutive UKALL trials to identify and validate a profile to predict outcome in high hyperdiploid B-ALL. The investigators defined a good-risk group (approximately 80% of high hyperdiploidy patients) that was associated with a more favorable prognosis. Good-risk patients had either trisomies of both chromosomes 17 and 18 or trisomy of one of these two chromosomes along with absence of trisomies of chromosomes 5 and 20. All other patients were defined as poor risk and had a less favorable outcome. End-induction MRD and copy number alterations (such as IKZF1 deletion) were prognostically significant within each hyperdiploid risk group.[
27 ]
Chromosomal translocations may be seen with high hyperdiploidy, and in those cases, patients are more appropriately risk-classified on the basis of the prognostic significance of the translocation. For instance, in one study, 8% of patients with the BCR::ABL1 fusion also had high hyperdiploidy,[
28 ] and the outcome of these patients (treated without tyrosine kinase inhibitors) was inferior to that observed in non-BCR::ABL1 high hyperdiploid patients.Certain patients with hyperdiploid ALL may have a hypodiploid clone that has doubled (masked hypodiploidy).[
29 ] Molecular technologies, such as single nucleotide polymorphism microarrays to detect widespread loss of heterozygosity, can be used to identify patients with masked hypodiploidy.[29 ] These cases may be interpretable based on the pattern of gains and losses of specific chromosomes (hyperdiploidy with two and four copies of chromosomes rather than three copies). These patients have an unfavorable outcome, similar to those with hypodiploidy.[30 ]Near triploidy (68–80 chromosomes) and near tetraploidy (>80 chromosomes) are much less common and appear to be biologically distinct from high hyperdiploidy.[
31 ] Unlike high hyperdiploidy, a high proportion of near tetraploid cases harbor a cryptic ETV6::RUNX1 fusion.[31 ,32 ,33 ] Near triploidy and tetraploidy were previously thought to be associated with an unfavorable prognosis, but later studies suggest that this may not be the case.[31 ,33 ]The genomic landscape of hyperdiploid ALL is characterized by variants in genes of the receptor tyrosine kinase (RTK)/RAS pathway in approximately one-half of cases. Genes encoding histone modifiers are also present in a recurring manner in a minority of cases. Analysis of variant profiles demonstrates that chromosomal gains are early events in the pathogenesis of hyperdiploid ALL and may occur in utero, while variants in RTK/RAS pathway genes are late events in leukemogenesis and are often subclonal.[
1 ,34 ] - A study combining experience from the Children's Cancer Group and the Pediatric Oncology Group (POG) found that patients with trisomies of chromosomes 4, 10, and 17 (triple trisomies) have a particularly favorable outcome.[
- Hypodiploidy (<44 chromosomes).
B-ALL cases with fewer than the normal number of chromosomes have been subdivided in various ways, with one report stratifying on the basis of modal chromosome number into the following four groups:[
30 ]- Near-haploid: 24 to 29 chromosomes (n = 46).
- Low-hypodiploid: 33 to 39 chromosomes (n = 26).
- High-hypodiploid: 40 to 43 chromosomes (n = 13).
- Near-diploid: 44 chromosomes (n = 54).
Near-haploid cases represent approximately 2% of NCI standard-risk and 2% of NCI high-risk pediatric B-ALL.[
1 ]Low-hypodiploid cases represent approximately 0.5% of NCI standard-risk and 2.6% of NCI high-risk pediatric B-ALL cases.[
1 ]Most patients with hypodiploidy are in the near-haploid and low-hypodiploid groups, and both of these groups have an elevated risk of treatment failure compared with nonhypodiploid cases.[
30 ,35 ] Patients with fewer than 44 chromosomes have a worse outcome than do patients with 44 or 45 chromosomes in their leukemic cells.[30 ] Several studies have shown that patients with high minimal residual disease (MRD) (≥0.01%) after induction do very poorly, with 5-year event-free survival (EFS) rates ranging from 25% to 47%. Although hypodiploid patients with low MRD after induction fare better (5-year EFS rates, 64%–75%), their outcomes are still inferior to most children with other types of ALL.[36 ,37 ,38 ]The recurring genomic alterations of near-haploid and low-hypodiploid ALL appear to be distinctive from each other and from other types of ALL.[
7 ] In near-haploid ALL, alterations targeting RTK signaling, RAS signaling, and IKZF3 are common.[39 ] In low-hypodiploid ALL, genetic alterations involving TP53, RB1, and IKZF2 are common. Importantly, the TP53 alterations observed in low-hypodiploid ALL are also present in nontumor cells in approximately 40% of cases, suggesting that these variants are germline and that low-hypodiploid ALL represents, in some cases, a manifestation of Li-Fraumeni syndrome.[7 ] Approximately two-thirds of patients with ALL and germline pathogenic TP53 variants have hypodiploid ALL.[40 ]
- High hyperdiploidy (51–65 chromosomes).
- Chromosomal translocations and gains/deletions of chromosomal segments.
- ETV6::RUNX1 fusion (t(12;21)(p13.2;q22.1)).
Fusion of the ETV6 gene on chromosome 12 to the RUNX1 gene on chromosome 21 is present in approximately 27% of NCI standard-risk and 10% of NCI high-risk pediatric B-ALL cases.[
1 ,32 ]The ETV6::RUNX1 fusion produces a cryptic translocation that is detected by methods such as FISH, rather than conventional cytogenetics, and it occurs most commonly in children aged 2 to 9 years.[
41 ,42 ] Hispanic children with ALL have a lower incidence of ETV6::RUNX1 fusions than do White children.[43 ]Reports generally indicate favorable EFS and overall survival (OS) rates in children with the ETV6::RUNX1 fusion; however, the prognostic impact of this genetic feature is modified by the following factors:[
44 ,45 ,46 ,47 ,48 ]; [16 ][Level of evidence B4]- Early response to treatment.
- NCI risk category (age and WBC count at diagnosis).
- Treatment regimen.
In one study of the treatment of newly diagnosed children with ALL, multivariate analysis of prognostic factors found age and leukocyte count, but not ETV6::RUNX1 fusion status, to be independent prognostic factors.[
44 ] However, another large trial only enrolled patients classified as having favorable-risk B-ALL, with low-risk clinical features, either trisomies of 4, 10, and 17 or ETV6::RUNX1 fusion, and end induction MRD less than 0.01%. Patients had a 5-year continuous complete remission rate of 93.7% and a 6-year OS rate of 98.2% for patients with ETV6::RUNX1.[16 ] It does not appear that the presence of secondary cytogenetic abnormalities, such as deletion of ETV6 (12p) or CDKN2A/B (9p), impacts the outcome of patients with the ETV6::RUNX1 fusion.[48 ,49 ]There is a higher frequency of late relapses in patients with ETV6::RUNX1 fusions compared with other relapsed B-ALL patients.[
44 ,50 ] Patients with the ETV6::RUNX1 fusion who relapse seem to have a better outcome than other relapse patients,[51 ] with an especially favorable prognosis for patients who relapse more than 36 months from diagnosis.[52 ] Some relapses in patients with ETV6::RUNX1 fusions may represent a new independent second hit in a persistent preleukemic clone (with the first hit being the ETV6::RUNX1 translocation).[53 ,54 ] - BCR::ABL1 fusion (t(9;22)(q34.1;q11.2); Ph+).
The BCR::ABL1 fusion leads to production of a BCR::ABL1 fusion protein with tyrosine kinase activity (see Figure 4).[
1 ] The BCR::ABL1 fusion occurs in approximately 2% of NCI standard-risk and 5% of NCI high-risk pediatric B-ALL cases.[1 ] The BCR::ABL1 fusion is also the leukemogenic driver for chronic myeloid leukemia (CML). The most common BCR breakpoint in CML is different from the most common BCR breakpoint in ALL. The breakpoint that typifies CML produces a larger fusion protein (termed p210) than the breakpoint most commonly observed for ALL (termed p190, a smaller fusion protein).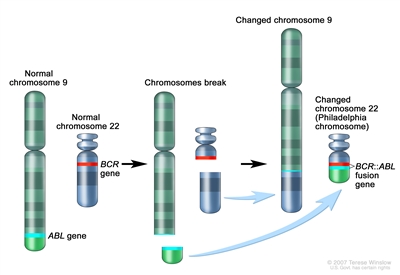
Figure 4. The Philadelphia chromosome is a translocation between the ABL1 oncogene (on the long arm of chromosome 9) and the BCR gene (on the long arm of chromosome 22), resulting in the fusion gene BCR::ABL1. BCR::ABL1 encodes an oncogenic protein with tyrosine kinase activity.Ph+ ALL is more common in older children with B-ALL and high WBC counts, with the incidence of the BCR::ABL1 fusions increasing to about 25% in young adults with ALL.
Historically, the BCR::ABL1 fusion was associated with an extremely poor prognosis (especially in those who presented with a high WBC count or had a slow early response to initial therapy), and its presence had been considered an indication for allogeneic hematopoietic stem cell transplant (HSCT) in patients in first remission.[
28 ,55 ,56 ,57 ] Inhibitors of the BCR::ABL1 tyrosine kinase, such as imatinib mesylate, are effective in patients with BCR::ABL1 ALL.[58 ] A study by the Children's Oncology Group (COG), which used intensive chemotherapy and concurrent imatinib mesylate given daily, demonstrated a 5-year EFS rate of 70% (± 12%), which was superior to the EFS rate of historical controls in the pre-tyrosine kinase inhibitor (imatinib mesylate) era. This result eliminated the recommendation of HSCT for patients with a good early response to chemotherapy using a tyrosine kinase inhibitor.[59 ,60 ]The International Consensus Classification of acute lymphoblastic leukemia/lymphoma from 2022 divides BCR::ABL1–positive B-ALL into two subtypes: cases with lymphoid-only involvement and cases with multilineage involvement.[
61 ] These subtypes differ in the timing of their transformation event. A multipotent progenitor serves as the target cell of origin for BCR::ABL1–positive B-ALL with multilineage involvement, and a later progenitor is the target cell of origin for BCR::ABL1–positive B-ALL with lymphoid-only involvement.- BCR::ABL1–positive B-ALL with lymphoid-only involvement is the predominate subtype. Only a minority of cases in children and adults have multilineage involvement (estimated at 15%–30%).[
62 ] - BCR::ABL1–positive B-ALL cases with lymphoid-only involvement and cases with multilineage involvement have similar clinical presentations and immunophenotypes. In addition, both subtypes commonly have the p190 fusion protein.[
62 ,63 ] - One way of distinguishing between patients with lymphoid-only and multilineage involvement is to detect the BCR::ABL1 fusion in normal non-ALL B cells, T cells, and myeloid cells.[
63 ] - A second way of distinguishing between patients with lymphoid-only and multilineage involvement is to detect quantitative differences in MRD levels (typically 1 log) using measures that quantify BCR::ABL1 DNA or RNA, compared with measures based on flow cytometry, real-time quantitative polymerase chain reaction (PCR), or next-generation sequencing (NGS) quantitation of leukemia-specific immunoglobulin (IG) or T-cell receptor (TCR) rearrangements.[
62 ,63 ,64 ]- For patients with lymphoid-only BCR::ABL1–positive B-ALL, MRD estimates for these methods will be correlated with each other.
- For patients with multilineage involvement BCR::ABL1–positive B-ALL, posttreatment MRD estimates based on detection of BCR::ABL1 DNA or RNA will often be higher than estimates based on flow cytometry or quantitation of leukemia-specific IG/TCR rearrangements.
- For patients with BCR::ABL1–positive B-ALL and multilineage involvement, levels of BCR::ABL1 transcripts and DNA may remain stable over time despite continued treatment with chemotherapy and tyrosine kinase inhibitors. In these situations, the persisting BCR::ABL1 DNA or RNA likely represents evidence of a residual preleukemic clone and not leukemia cells. Therefore, the term MRD is a misnomer.
- A corollary of the difference in MRD detection by methods based on BCR::ABL1 DNA or RNA detection versus MRD detection based on flow cytometry or IG/TCR rearrangements is that the latter methods provide more reliable prognostication.[
62 ,64 ,65 ] For example, the presence of MRD by BCR::ABL1 DNA or RNA detection in the absence of MRD detection by IG/TCR rearrangements does not confer inferior prognosis. - Based on the limited numbers of patients studied to date, prognosis appears similar in both adults and children with lymphoid-only versus multilineage involvement BCR::ABL1–positive B-ALL.[
62 ,64 ] - There are case reports of patients with multilineage involvement BCR::ABL1–positive B-ALL who relapse years from their initial diagnosis. In addition, their relapsed leukemia has the same BCR::ABL1 breakpoint as their initial leukemia, but it has a different IG/TCR rearrangement.[
64 ] These case reports suggest that patients with multilineage BCR::ABL1–positive B-ALL are at risk of a second leukemogenic event, leading to a second BCR::ABL1 leukemia. - There is no evidence that a specific monitoring schedule or prolonged treatment with a tyrosine kinase inhibitor provides clinical benefit for patients with multilineage involvement BCR::ABL1–positive B-ALL who have maintained presence of BCR::ABL1 transcripts or DNA at the completion of a standard-duration course of leukemia therapy.
- BCR::ABL1–positive B-ALL with lymphoid-only involvement is the predominate subtype. Only a minority of cases in children and adults have multilineage involvement (estimated at 15%–30%).[
- KMT2A-rearranged ALL (t(v;11q23.3)).
Rearrangements involving the KMT2A gene with more than 100 translocation partner genes result in the production of fusion oncoproteins. KMT2A gene rearrangements occur in up to 80% of infants with ALL. Beyond infancy, approximately 1% of NCI standard-risk and 4% of NCI high-risk pediatric B-ALL cases have KMT2A rearrangements.[
1 ]These rearrangements are generally associated with an increased risk of treatment failure, particularly in infants.[
66 ,67 ,68 ,69 ] The KMT2A::AFF1 fusion (t(4;11)(q21;q23)) is the most common rearrangement involving the KMT2A gene in children with ALL and occurs in approximately 1% to 2% of childhood ALL.[67 ,70 ]Patients with KMT2A::AFF1 fusions are usually infants with high WBC counts. These patients are more likely than other children with ALL to have central nervous system (CNS) disease and to have a poor response to initial therapy.[
71 ] While both infants and adults with the KMT2A::AFF1 fusion are at high risk of treatment failure, children with the KMT2A::AFF1 fusion appear to have a better outcome.[66 ,67 ,72 ] Irrespective of the type of KMT2A gene rearrangement, infants with KMT2A-rearranged ALL have much worse event-free survival rates than non-infant pediatric patients with KMT2A-rearranged ALL.[66 ,67 ,72 ]Whole-genome sequencing has determined that cases of infant ALL with KMT2A gene rearrangements have frequent subclonal NRAS or KRAS variants and few additional genomic alterations, none of which have clear clinical significance.[
11 ,73 ] Deletion of the KMT2A gene has not been associated with an adverse prognosis.[74 ]Of interest, the KMT2A::MLLT1 fusion (t(11;19)(q23;p13.3)) occurs in approximately 1% of ALL cases and occurs in both early B-lineage ALL and T-ALL.[
75 ] Outcome for infants with the KMT2A::MLLT1 fusion is poor, but outcome appears relatively favorable in older children with T-ALL and the KMT2A::MLLT1 fusion.[75 ] - TCF3::PBX1 fusion (t(1;19)(q23;p13.3)) and TCF3::HLF fusion (t(17;19)(q22;p13)).
Fusion of the TCF3 gene on chromosome 19 to the PBX1 gene on chromosome 1 is present in approximately 4% of NCI standard-risk and 5% of NCI high-risk pediatric B-ALL cases.[
1 ,76 ,77 ] The TCF3::PBX1 fusion may occur as either a balanced translocation or as an unbalanced translocation and is the primary recurring genomic alteration of the pre-B–ALL immunophenotype (cytoplasmic immunoglobulin positive).[78 ] Black children are relatively more likely than White children to have pre-B–ALL with the TCF3::PBX1 fusion.[79 ]The TCF3::PBX1 fusion had been associated with inferior outcome in the context of antimetabolite-based therapy,[
80 ] but the adverse prognostic significance was largely negated by more aggressive multiagent therapies.[77 ,81 ] More specifically, in a trial conducted by St. Jude Children's Research Hospital (SJCRH) in which all patients were treated without cranial radiation, patients with the TCF3::PBX1 fusion had an overall outcome comparable to children lacking this translocation, but with a higher risk of CNS relapse and a lower rate of bone marrow relapse, suggesting that more intensive CNS therapy may be needed for these patients.[82 ,83 ]The TCF3::HLF fusion occurs in less than 1% of pediatric ALL cases. ALL with the TCF3::HLF fusion is associated with disseminated intravascular coagulation and hypercalcemia at diagnosis. Outcome is very poor for children with the TCF3::HLF fusion, with a literature review noting mortality for 20 of 21 cases reported.[
84 ] In addition to the TCF3::HLF fusion, the genomic landscape of this ALL subtype was characterized by deletions in genes involved in B-cell development (PAX5, BTG1, and VPREB1) and by variants in RAS pathway genes (NRAS, KRAS, and PTPN11).[78 ] - DUX4-rearranged ALL with frequent ERG deletions.
Approximately 3% of NCI standard-risk and 6% of NCI high-risk pediatric B-ALL patients have a rearrangement involving DUX4 that leads to its overexpression.[
1 ,5 ,6 ] East Asian ancestry was linked to an increased prevalence of DUX4-rearranged ALL (favorable).[85 ] The most common rearrangement produces IGH::DUX4 fusions, with ERG::DUX4 fusions also observed.[86 ]DUX4-rearranged cases show a distinctive gene expression pattern that was initially identified as being associated with focal deletions in ERG,[86 ,87 ,88 ,89 ] and one-half to more than two-thirds of these cases have focal intragenic deletions involving ERG that are not observed in other ALL subtypes.[5 ,86 ]ERG deletions often appear to be clonal, but using sensitive detection methodology, it appears that most cases are polyclonal.[86 ]IKZF1 alterations are observed in 20% to 40% of DUX4-rearranged ALL.[5 ,6 ]ERG deletion connotes an excellent prognosis, with OS rates exceeding 90%. Even when the IZKF1 deletion is present, prognosis remains highly favorable.[
87 ,88 ,89 ,90 ] While patients with DUX4-rearranged ALL have an overall favorable prognosis, there is uncertainty as to whether this applies to both ERG-deleted and ERG-intact cases. In a study of 50 patients with DUX4-rearranged ALL, patients with an ERG deletion detected by genomic PCR (n = 33) had a more favorable EFS rate of approximately 90% than did patients with intact ERG (n = 17), with an EFS rate of approximately 70%.[88 ] - MEF2D-rearranged ALL.
Gene fusions involving MEF2D, a transcription factor that is expressed during B-cell development, are observed in approximately 0.3% of NCI standard-risk and 3% of NCI high-risk pediatric B-ALL cases.[
1 ,91 ,92 ]Although multiple fusion partners may occur, most cases involve BCL9, which is located on chromosome 1q21, as is MEF2D.[
91 ,93 ] The interstitial deletion producing the MEF2D::BCL9 fusion is too small to be detected by conventional cytogenetic methods. Cases with MEF2D gene fusions show a distinctive gene expression profile, except for rare cases with MEF2D::CSFR1 that have a BCR::ABL1-like gene expression profile.[91 ,94 ]The median age at diagnosis for cases of MEF2D-rearranged ALL in studies that included both adult and pediatric patients was 12 to 14 years.[
91 ,92 ] For 22 children with MEF2D-rearranged ALL enrolled in a high-risk ALL clinical trial, the 5-year EFS rate was 72% (standard error, ± 10%), which was inferior to that for other patients.[91 ] - ZNF384-rearranged ALL.
ZNF384 is a transcription factor that is rearranged in approximately 0.3% of NCI standard-risk and 2.7% of NCI high-risk pediatric B-ALL cases.[
1 ,91 ,95 ,96 ]East Asian ancestry was associated with an increased prevalence of ZNF384.[
85 ] Multiple fusion partners for ZNF384 have been reported, including ARID1B, CREBBP, EP300, SMARCA2, TAF15, and TCF3. Regardless of the fusion partner, ZNF384-rearranged ALL cases show a distinctive gene expression profile.[91 ,95 ,96 ]ZNF384 rearrangement does not appear to confer independent prognostic significance.[91 ,95 ,96 ] However, within the subset of patients with ZNF384 rearrangements, patients with EP300::ZNF384 fusions have lower relapse rates than patients with other ZNF384 fusion partners.[97 ] The immunophenotype of B-ALL with ZNF384 rearrangement is characterized by weak or negative CD10 expression, with expression of CD13 and/or CD33 commonly observed.[95 ,96 ] Cases of mixed phenotype acute leukemia (MPAL) (B/myeloid) that have ZNF384 gene fusions have been reported,[98 ,99 ] and a genomic evaluation of MPAL found that ZNF384 gene fusions were present in approximately one-half of B/myeloid cases.[100 ] - NUTM1-rearranged B-ALL.
NUTM1-rearranged B-ALL is most commonly observed in infants, representing 3% to 5% of overall cases of B-ALL in this age group and approximately 20% of infant B-ALL cases lacking the KMT2A rearrangement.[
101 ] The frequency of NUTM1 rearrangement is lower in children after infancy (<1% of cases).[1 ,101 ]The NUTM1 gene is located on chromosome 15q14, and some cases of B-ALL with NUTM1 rearrangements show chromosome 15q aberrations, but other cases are cryptic and have no cytogenetic abnormalities.[
102 ] RNA sequencing, as well as break-apart FISH, can be used to detect the presence of the NUTM1 rearrangement.[101 ]The NUTM1 rearrangement appears to be associated with a favorable outcome.[
101 ,103 ] Among 35 infants with NUTM1-rearranged B-ALL who were treated on Interfant protocols, all patients achieved remission and no relapses were observed.[101 ] For the 32 children older than 12 months with NUTM1-rearranged B-ALL, the 4-year EFS and OS rates were 92% and 100%, respectively. - IGH::IL3 fusion (t(5;14)(q31.1;q32.3)).
This entity is included in the 2016 revision of the World Health Organization (WHO) classification of tumors of the hematopoietic and lymphoid tissues.[
104 ] The finding of t(5;14)(q31.1;q32.3) in patients with ALL and hypereosinophilia in the 1980s was followed by the identification of the IGH::IL3 fusion as the underlying genetic basis for the condition.[105 ,106 ] The joining of the IGH locus to the promoter region of the IL3 gene leads to dysregulation of IL3 expression.[107 ] Cytogenetic abnormalities in children with ALL and eosinophilia are variable, with only a subset resulting from the IGH::IL3 fusion.[108 ]The number of cases of IGH::IL3 ALL described in the published literature is too small to assess the prognostic significance of the IGH::IL3 fusion. Diagnosis of cases of IGH::IL3 ALL may be delayed because the ALL clone in the bone marrow may be small, and because it can present with hypereosinophilia in the absence of cytopenias and circulating blasts.[
104 ] - Intrachromosomal amplification of chromosome 21 (iAMP21).
iAMP21 occurs in approximately 5% of NCI standard-risk and 7% of NCI high-risk pediatric B-ALL cases.[
1 ] iAMP21 is generally diagnosed using FISH and is defined by the presence of greater than or equal to five RUNX1 signals per cell (or ≥3 extra copies of RUNX1 on a single abnormal chromosome).[104 ] iAMP21 can also be identified by chromosomal microarray analysis. Uncommonly, iAMP21 with an atypical genomic pattern (e.g., amplification of the genomic region but with less than 5 RUNX1 signals or having at least 5 RUNX1 signals with some located apart from the abnormal iAMP21-chromosome) is identified by microarray but not RUNX1 FISH.[109 ] The prognostic significance of iAMP21 defined only by microarray has not been characterized.iAMP21 is associated with older age (median, approximately 10 years), presenting WBC count of less than 50 × 109 /L, a slight female preponderance, and high end-induction MRD.[
110 ,111 ,112 ] Analysis of variant signatures indicates that gene amplifications in iAMP21 occur later in leukemogenesis, which is in contrast to those of hyperdiploid ALL that can arise early in life and even in utero.[1 ]The United Kingdom Acute Lymphoblastic Leukaemia (UKALL) clinical trials group initially reported that the presence of iAMP21 conferred a poor prognosis in patients treated in the MRC ALL 97/99 trial (5-year EFS rate, 29%).[
17 ] In their subsequent trial (UKALL2003 [NCT00222612]), patients with iAMP21 were assigned to a more intensive chemotherapy regimen and had a markedly better outcome (5-year EFS rate, 78%).[111 ] Similarly, the COG has reported that iAMP21 was associated with a significantly inferior outcome in NCI standard-risk patients (4-year EFS rate, 73% for iAMP21 vs. 92% in others), but not in NCI high-risk patients (4-year EFS rate, 73% vs. 80%).[110 ] On multivariate analysis, iAMP21 was an independent predictor of inferior outcome only in NCI standard-risk patients.[110 ] The results of the UKALL2003 and COG studies suggest that treatment of iAMP21 patients with high-risk chemotherapy regimens abrogates its adverse prognostic significance and obviates the need for HSCT in first remission.[112 ] - PAX5 alterations.
Gene expression analysis identified two distinctive ALL subsets with PAX5 genomic alterations, called PAX5alt and PAX5 p.P80R (NP_057953.1).[
113 ] The alterations in the PAX5alt subtype included rearrangements, sequence variants, and focal intragenic amplifications.PAX5alt. PAX5 rearrangements have been reported to represent approximately 3% of NCI standard-risk and 11% of NCI high-risk pediatric B-ALL cases.[
1 ] More than 20 partner genes for PAX5 have been described,[113 ] with PAX5::ETV6, the primary genomic alteration in dic(9;12)(p13;p13),[114 ] being the most common gene fusion.[113 ]Intragenic amplification of PAX5 was identified in approximately 1% of B-ALL cases, and it was usually detected in cases lacking known leukemia-driver genomic alterations.[
115 ] Cases with PAX5 amplification show male predominance (66%), with most (55%) having NCI high-risk status. For a cohort of patients with PAX5 amplification diagnosed between 1993 and 2015, the 5-year EFS rate was 49% (95% confidence interval [CI], 36%–61%), and the OS rate was 67% (95% CI, 54%–77%), suggesting a relatively poor prognosis for patients with this B-ALL subtype.PAX5 p.P80R (NP_057953.1). PAX5 with a p.P80R variant shows a gene expression profile distinctive from that of other cases with PAX5 alterations.[
113 ] Cases with PAX5 p.P80R represent approximately 0.3% of NCI standard-risk and 1.8% of NCI high-risk pediatric B-ALL.[1 ] PAX5 p.P80R B-ALL appears to occur more frequently in the adolescent and young adult (AYA) and adult populations (3.1% and 4.2%, respectively).[113 ]Outcome for the pediatric patients with PAX5 p.P80R and PAX5alt treated in a COG clinical trial appears to be intermediate (5-year EFS rate, approximately 75%).[
113 ]PAX5alt rearrangements have also been detected in infant patients with ALL, with a reported outcome similar to KMT2A-rearranged infant ALL.[103 ] - BCR::ABL1-like (Ph-like).
BCR::ABL1-negative patients with a gene expression profile similar to BCR::ABL1-positive patients have been referred to as Ph-like,[
116 ,117 ,118 ] and are now referred to as BCR::ABL1-like.[18 ] This occurs in 10% to 20% of pediatric B-ALL patients, increasing in frequency with age, and has been associated with an IKZF1 deletion or variant.[1 ,8 ,116 ,117 ,119 ,120 ]Retrospective analyses have indicated that patients with BCR::ABL1-like ALL have a poor prognosis.[
4 ,116 ] In one series, the 5-year EFS rate for NCI high-risk children and adolescents with BCR::ABL1-like ALL was 58% and 41%, respectively.[4 ] While it is more frequent in older and higher-risk patients, the BCR::ABL1-like subtype has also been identified in NCI standard-risk patients. In a COG study, 13.6% of 1,023 NCI standard-risk B-ALL patients were found to have BCR::ABL1-like ALL; these patients had an inferior EFS rate compared with non–BCR::ABL1-like standard-risk patients (82% vs. 91%), although no difference in OS rate (93% vs. 96%) was noted.[121 ] In one study of 40 BCR::ABL1-like patients, the adverse prognostic significance of this subtype appeared to be abrogated when patients were treated with risk-directed therapy on the basis of MRD levels.[122 ]The hallmark of BCR::ABL1-like ALL is activated kinase signaling, with approximately 35% to 50% containing CRLF2 genomic alterations [
1 ,118 ,123 ] and half of those cases containing concomitant JAK variants.[124 ]Many of the remaining cases of BCR::ABL1-like ALL have been noted to have a series of translocations involving tyrosine-kinase encoding ABL-class fusion genes, including ABL1, ABL2, CSF1R, and PDGFRB.[
4 ,119 ,125 ] Fusion proteins from these gene combinations have been noted in some cases to be transformative and have responded to tyrosine kinase inhibitors both in vitro and in vivo,[119 ] suggesting potential therapeutic strategies for these patients.BCR::ABL1-like ALL cases with non-CRLF2 genomic alterations represent approximately 3% of NCI standard-risk and 8% of NCI high-risk pediatric B-ALL cases.[
1 ] In a retrospective study of 122 pediatric patients (aged 1–18 years) with ABL-class fusions (all treated without tyrosine kinase inhibitors), the 5-year EFS rate was 59%, and the OS rate was 76%.[126 ]Approximately 9% of BCR::ABL1-like ALL cases result from rearrangements that lead to overexpression of a truncated erythropoietin receptor (EPOR).[
127 ] The C-terminal region of the receptor that is lost is the region that is altered in primary familial congenital polycythemia and that controls stability of the EPOR. The portion of the EPOR remaining is sufficient for JAK-STAT activation and for driving leukemia development. Point variants in kinase genes, aside from those in JAK1 and JAK2, are uncommon in patients with BCR::ABL1-like ALL.[8 ]CRLF2. Genomic alterations in CRLF2, a cytokine receptor gene located on the pseudoautosomal regions of the sex chromosomes, have been identified in 5% to 10% of cases of B-ALL. These alterations represent approximately 50% of cases of BCR::ABL1-like ALL.[
128 ,129 ,130 ] The chromosomal abnormalities that commonly lead to CRLF2 overexpression include translocations of the IGH locus (chromosome 14) to CRLF2 and interstitial deletions in pseudoautosomal regions of the sex chromosomes, resulting in a P2RY8::CRLF2 fusion.[8 ,123 ,128 ,129 ] These two genomic alterations are associated with distinctive clinical and biological characteristics.BCR::ABL1-like B-ALL with CRLF2 genomic alterations is observed in approximately 2% of NCI standard-risk and 5% of NCI high-risk pediatric B-ALL cases.[
1 ]ALL with genomic alterations in CRLF2 occurs at a higher incidence in children with Hispanic or Latino genetic ancestry [
123 ,131 ] and American Indian genetic ancestry.[85 ] In a study of 205 children with high-risk B-ALL, 18 of 51 (35.3%) Hispanic or Latino patients had CRLF2 rearrangements, compared with 11 of 154 (7.1%) cases of other declared ethnicity.[123 ] In a second study, only the frequency of IGH::CRLF2 fusions was increased in Hispanic or Latino children compared with non-Hispanic or non-Latino children with B-ALL (12% vs. 2.7%).[131 ] In this study, the percentage of B-ALL with P2RY8::CRLF2 fusions was approximately 6% and was not affected by ethnicity.The P2RY8::CRLF2 fusion is observed in 70% to 75% of pediatric patients with CRLF2 genomic alterations, and it occurs in younger patients (median age, approximately 4 years vs. 14 years for patients with IGH::CRLF2).[
132 ,133 ]P2RY8::CRLF2 occurs not infrequently with established chromosomal abnormalities (e.g., hyperdiploidy, iAMP21, dic(9;20)), while IGH::CRLF2 is generally mutually exclusive with known cytogenetic subgroups. CRLF2 genomic alterations are observed in approximately 60% of patients with Down syndrome and ALL, with P2RY8::CRLF2 fusions being more common than IGH::CRLF2 (approximately 80%–85% vs. 15%–20%).[129 ,132 ]IGH::CRLF2 and P2RY8::CRLF2 commonly occur as an early event in B-ALL development and show clonal prevalence.[
134 ] However, in some cases they appear to be a late event and show subclonal prevalence.[134 ] Loss of the CRLF2 genomic abnormality in some cases at relapse confirms the subclonal nature of the alteration in these cases.[132 ,135 ]CRLF2 abnormalities are strongly associated with the presence of IKZF1 deletions. Deletions of IKZF1 are more common in cases with IGH::CRLF2 fusions than in cases with P2RY8::CRLF2 fusions.[
133 ] Other recurring genomic alterations found in association with CRLF2 alterations include deletions in genes associated with B-cell differentiation (e.g., PAX5, BTG1, EBF1, etc.) and cell cycle control (CDKN2A), as well as genomic alterations activating JAK-STAT pathway signaling (e.g., IL7R and JAK variants).[4 ,123 ,124 ,129 ,136 ]Although the results of several retrospective studies suggest that CRLF2 abnormalities may have adverse prognostic significance in univariate analyses, most do not find this abnormality to be an independent predictor of outcome.[
123 ,128 ,129 ,137 ,138 ] For example, in a large European study, increased expression of CRLF2 was not associated with unfavorable outcome in multivariate analysis, while IKZF1 deletion and BCR::ABL1-like expression signatures were associated with unfavorable outcome.[120 ] Controversy exists about whether the prognostic significance of CRLF2 abnormalities should be analyzed on the basis of CRLF2 overexpression or on the presence of CRLF2 genomic alterations.[137 ,138 ] - IKZF1 deletions.
IKZF1 deletions, including deletions of the entire gene and deletions of specific exons, are present in approximately 15% of B-ALL cases. Less commonly, IKZF1 can be inactivated by deleterious point variants.[
117 ]Cases with IKZF1 deletions tend to occur in older children, have a higher WBC count at diagnosis, and are therefore more common in NCI high-risk patients than in NCI standard-risk patients.[
2 ,117 ,136 ,139 ,140 ] A high proportion of BCR::ABL1-positive cases have a deletion of IKZF1,[3 ,136 ] and ALL arising in children with Down syndrome appears to have elevated rates of IKZF1 deletions.[141 ]IKZF1 deletions are also common in cases with CRLF2 genomic alterations and in BCR::ABL1-like ALL cases.[87 ,116 ,136 ]Multiple reports have documented the adverse prognostic significance of an IKZF1 deletion, and most studies have reported that this deletion is an independent predictor of poor outcome in multivariate analyses.[
87 ,116 ,117 ,120 ,136 ,142 ,143 ,144 ,145 ,146 ,147 ,148 ,149 ]; [150 ][Level of evidence B4] However, the prognostic significance of IKZF1 may not apply equally across ALL biological subtypes, as illustrated by the apparent lack of prognostic significance in patients with ERG deletions.[87 ,88 ,89 ] Similarly, the prognostic significance of the IKZF1 deletion also appeared to be minimized in a cohort of COG patients with DUX4-rearranged ALL and with ERG transcriptional dysregulation that frequently occurred by ERG deletion.[6 ] The Associazione Italiana di Ematologia e Oncologia Pediatrica–Berlin-Frankfurt-Münster group reported that IKZF1 deletions were significant adverse prognostic factors only in B-ALL patients with high end-induction MRD and in whom co-occurrence of deletions of CDKN2A, CDKN2B, PAX5, or PAR1 (in the absence of ERG deletion) were identified.[151 ] The poor prognosis associated with IKZF1 alterations appears to be enhanced by the concomitant finding of deletion of 22q11.22. In a study of 1,310 patients with B-ALL, approximately one-half of the patients with IKZF1 alterations also had deletion of 22q11.22. The 5-year EFS rate was 43.3% for those with both abnormalities, compared with 68.5% for patients with IKZF1 alterations and wild-type 22q11.22 (P < .001).[152 ]There are few published results of changing therapy on the basis of IKZF1 gene status. The Malaysia-Singapore group published results of two consecutive trials. In the first trial (MS2003), IKZF1 status was not considered in risk stratification, while in the subsequent trial (MS2010), IKZF1-deleted patients were excluded from the standard-risk group. Thus, more IKZF1-deleted patients in the MS2010 trial received intensified therapy. Patients with IKZF1-deleted ALL had improved outcomes in MS2010 compared with patients in MS2003, but interpretation of this observation is limited by other changes in risk stratification and therapeutic differences between the two trials.[
153 ][Level of evidence B4]In the Dutch ALL11 study, patients with IKZF1 deletions had maintenance therapy extended by 1 year, with the goal of improving outcomes.[
154 ] The landmark analysis demonstrated an almost threefold reduction in relapse rate and an improvement in the 2-year EFS rate (from 74.4% to 91.2%), compared with historical controls. - MYC-rearranged ALL (8q24).
MYC gene rearrangements are a rare but recurrent finding in pediatric patients with B-ALL. Patients with rearrangements of the MYC gene and the IGH2, IGK, and IGL genes at 14q32, 2p12, and 22q11.2, respectively, have been reported.[
155 ,156 ,157 ] The lymphoblasts typically exhibit a precursor B-cell immunophenotype, with a French-American-British (FAB) L2 or L3 morphology, with no expression of surface immunoglobulin and kappa or lambda light chains. Concurrent MYC gene rearrangements have been observed along with additional cytogenetic rearrangements such as IGH::BCL2 or KMT2A.[157 ] Patients reported in the literature have been variably treated with ALL therapy or with mature B leukemia/lymphoma treatment protocols, and the optimal treatment for this patient group remains uncertain.[157 ]
- ETV6::RUNX1 fusion (t(12;21)(p13.2;q22.1)).
T-ALL cytogenetics/genomics
T-ALL is characterized by genomic alterations leading to activation of transcriptional programs related to T-cell development and by a high frequency of cases (approximately 60%) with variants in NOTCH1 and/or FBXW7 that result in activation of the NOTCH1 pathway.[
In Figure 5 below, pediatric T-ALL cases are divided into 10 molecular subtypes based on their RNA expression and gene variant status. These cases were derived from patients enrolled in SJCRH and COG clinical trials.[
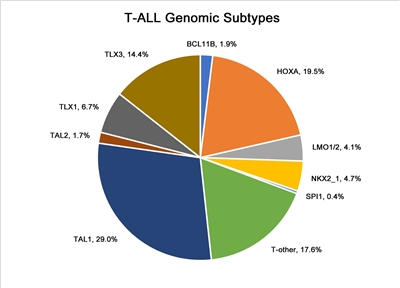
Figure 5. Genomic subtypes of T-ALL. The figure represents data from 466 children, adolescents, and young adults diagnosed with T-ALL and enrolled in St. Jude Children's Research Hospital or Children's Oncology Group clinical trials. Adapted from Brady SW, Roberts KG, Gu Z, et al.: The genomic landscape of pediatric acute lymphoblastic leukemia. Nature Genetics 54: 1376-1389, 2022.
- Notch pathway signaling.
Notch pathway signaling is commonly activated by NOTCH1 and FBXW7 gene variants in T-ALL, and these are the most commonly altered genes in pediatric T-ALL.[
158 ,163 ]NOTCH1-activating gene variants occur in approximately 50% to 60% of T-ALL cases, and FBXW7-inactivating gene variants occur in approximately 15% of cases. Approximately 60% of T-ALL cases have Notch pathway activation by variants in at least one of these genes.[164 ,165 ]The prognostic significance of NOTCH1 and FBXW7 variants may be modulated by genomic alterations in RAS and PTEN. The French Acute Lymphoblastic Leukaemia Study Group (FRALLE) and the Group for Research on Adult Acute Lymphoblastic Leukemia reported that patients having altered NOTCH1 or FBXW7 and wild-type PTEN and RAS constituted a favorable-risk group (i.e., low-risk group), while patients with PTEN or RAS variants, regardless of NOTCH1 and FBXW7 status, have a significantly higher risk of treatment failure (i.e., high-risk group).[
166 ,167 ] In the FRALLE study, the 5-year disease-free survival rate was 88% for the genetic low-risk group of patients and 60% for the genetic high-risk group of patients.[166 ] However, using the same criteria to define the genetic risk group, the Dana-Farber Cancer Institute consortium was unable to replicate these results. They reported a 5-year EFS rate of 86% for genetic low-risk patients and 79% for the genetic high-risk patients, a difference that was not statistically significant (P = .26).[165 ] - Chromosomal translocations.
Multiple chromosomal translocations have been identified in T-ALL that lead to deregulated expression of the target genes. These chromosome rearrangements fuse genes encoding transcription factors (e.g., TAL1, TAL2, LMO1, LMO2, LYL1, TLX1, TLX3, NKX2-I, HOXA, and MYB) to one of the T-cell receptor loci (or to other genes) and result in deregulated expression of these transcription factors in leukemia cells.[
158 ,159 ,168 ,169 ,170 ,171 ,172 ] These translocations are often not apparent by examining a standard karyotype, but can be identified using more sensitive screening techniques, including FISH or PCR.[159 ] Variants in a noncoding region near the TAL1 gene that produce a super-enhancer upstream of TAL1 represent nontranslocation genomic alterations that can also activate TAL1 transcription to induce T-ALL.[161 ]Translocations resulting in chimeric fusion proteins are also observed in T-ALL.[
166 ]- A NUP214::ABL1 fusion has been noted in 4% to 6% of T-ALL cases and is observed in both adults and children, with a male predominance.[
173 ,174 ,175 ] The fusion is cytogenetically cryptic and is seen in FISH on amplified episomes or, more rarely, as a small homogeneous staining region.[175 ] T-ALL may also uncommonly show ABL1 fusion proteins with other gene partners (e.g., ETV6, BCR, and EML1).[175 ] ABL tyrosine kinase inhibitors, such as imatinib or dasatinib, may demonstrate therapeutic benefits in this T-ALL subtype,[173 ,174 ,176 ] although clinical experience with this strategy is very limited.[177 ,178 ,179 ] - Gene fusions involving SPI1 (encoding the transcription factor PU.1) were reported in 4% of Japanese children with T-ALL.[
180 ] Fusion partners included STMN1 and TCF7. T-ALL cases with SPI1 fusions had a particularly poor prognosis; six of seven affected individuals died within 3 years of diagnosis of early relapse. - BCL11B is a zinc finger transcription factor that plays a dual role as a transcription activator and repressor. It is known to play a critical role in T-cell differentiation. In T-ALL, the BCL11B gene is involved in a t(5;14)(q35;q32) translocation where a distal BCL11B enhancer drives aberrant expression of TLX3 (or NKX2-5).[
181 ] In the process of donating its enhancer, one allele of BCL11B is inactivated. However, the resulting haploinsufficient state itself may also play a role in tumor pathogenesis. The role of BCL11B as a tumor suppressor gene is supported by the finding that about 16% of patients have T-ALL that harbors deletions or missense variants.[158 ,182 ] As described in the sections for early T-cell precursor (ETP) and T/myeloid mixed phenotype acute leukemia (T/M MPAL), BCL11B may also be leukemogenic through overexpression. - Other recurring gene fusions in T-ALL patients include those involving MLLT10, KMT2A, NUP214, and NUP98.[
158 ,162 ]
- A NUP214::ABL1 fusion has been noted in 4% to 6% of T-ALL cases and is observed in both adults and children, with a male predominance.[
- Ploidy.
- Recurrent abnormalities in chromosome number are much less common in T-ALL than in B-ALL. One study included 2,250 pediatric patients with T-ALL who were treated in Associazione Italiana di Ematologia e Oncologia Pediatrica/Berlin-Frankfurt-Münster protocols. The study found that near tetraploidy (DNA index, 1.79–2.28 or 81–103 chromosomes), observed in 1.4% of patients, was associated with favorable disease features and outcomes.[
183 ]
- Recurrent abnormalities in chromosome number are much less common in T-ALL than in B-ALL. One study included 2,250 pediatric patients with T-ALL who were treated in Associazione Italiana di Ematologia e Oncologia Pediatrica/Berlin-Frankfurt-Münster protocols. The study found that near tetraploidy (DNA index, 1.79–2.28 or 81–103 chromosomes), observed in 1.4% of patients, was associated with favorable disease features and outcomes.[
Early T-cell precursor (ETP) ALL cytogenetics/genomics
Detailed molecular characterization of ETP ALL showed this entity to be highly heterogeneous at the molecular level, with no single gene affected by variant or copy number alteration in more than one-third of cases.[
Studies have found that the absence of biallelic deletion of the TCR-gamma locus (ABD), as detected by comparative genomic hybridization and/or quantitative DNA-PCR, was associated with early treatment failure in patients with T-ALL.[
Allele-specific, generally high expression of BCL11B plays an oncogenic role in a subset of cases identified as ETP ALL (7 of 58 in one study) as well as in up to 30% to 40% of lineage ambiguous leukemia T/M mixed phenotype acute leukemia (T/M MPAL).[
- One such alteration is t(2;14)(q22;q32), which produces an in-frame ZEB2::BCL11B fusion gene.
- Other structural variants leading to allele-specific deregulated BCL11B expression include structural variants that juxtapose regulatory sequences of active genes (e.g., ARID1B [chromosome 6], BENC [chromosome 7], and CDK6 [chromosome 7]) upstream or downstream of the BCL11B locus leading to aberrant expression in a process called enhancer hijacking.
- Finally, in about 20% of cases with deregulated BCL11B expression, a translocation cannot be identified. In many such cases, amplification of a downstream enhancer, BCL11B enhancer tandem amplification (BETA), leads to BCL11B promoter driven transcription.
- There is a high prevalence of FLT3 alterations and JAK/STAT activation in acute leukemias driven by genomic alterations leading to BCL11B expression.[
187 ]
Mixed phenotype acute leukemia (MPAL) cytogenetics/genomics
For acute leukemias of ambiguous lineage, the WHO classification system is summarized in Table 3.[
| Condition | Definition |
|---|---|
| MPAL = mixed phenotype acute leukemia; NOS = not otherwise specified. | |
| a Adapted from Béné MC: Biphenotypic, bilineal, ambiguous or mixed lineage: strange leukemias! Haematologica 94 (7): 891-3, 2009.[ |
|
| Acute undifferentiated leukemia | Acute leukemia that does not express any marker considered specific for either lymphoid or myeloid lineage |
| MPAL withBCR::ABL1(t(9;22)(q34;q11.2)) | Acute leukemia meeting the diagnostic criteria for MPAL in which the blasts also have the (9;22) translocation or theBCR::ABL1rearrangement |
| MPAL withKMT2A(t(v;11q23)) | Acute leukemia meeting the diagnostic criteria for MPAL in which the blasts also have a translocation involving theKMT2Agene |
| MPAL, B/myeloid, NOS (B/M MPAL) | Acute leukemia meeting the diagnostic criteria for assignment to both B and myeloid lineage, in which the blasts lack genetic abnormalities involvingBCR::ABL1orKMT2A |
| MPAL, T/myeloid, NOS (T/M MPAL) | Acute leukemia meeting the diagnostic criteria for assignment to both T and myeloid lineage, in which the blasts lack genetic abnormalities involvingBCR::ABL1orKMT2A |
| MPAL, B/myeloid, NOS—rare types | Acute leukemia meeting the diagnostic criteria for assignment to both B and T lineage |
| Other ambiguous lineage leukemias | Natural killer–cell lymphoblastic leukemia/lymphoma |
| Lineage | Criteria |
|---|---|
| a Adapted from Arber et al.[ |
|
| b Strong defined as equal to or brighter than the normal B or T cells in the sample. | |
| Myeloid lineage | Myeloperoxidase (flow cytometry, immunohistochemistry, or cytochemistry);or monocytic differentiation (at least two of the following: nonspecific esterase cytochemistry, CD11c, CD14, CD64, lysozyme) |
| T lineage | Strongb cytoplasmic CD3 (with antibodies to CD3 epsilon chain);or surface CD3 |
| B lineage | Strongb CD19 with at least one of the following strongly expressed: CD79a, cytoplasmic CD22, or CD10;or weak CD19 with at least two of the following strongly expressed: CD79a, cytoplasmic CD22, or CD10 |
The classification system for MPAL includes two entities that are defined by their primary molecular alteration: MPAL with BCR::ABL1 translocation and MPAL with KMT2A rearrangement. The genomic alterations associated with the MPAL, B/myeloid, NOS (B/M MPAL) and MPAL, T/myeloid, NOS (T/M MPAL) entities are distinctive, as described below:
- B/M MPAL.
- Among 115 MPAL cases for which genomic characterization was performed, 35 (30%) were B/M MPAL. There were an additional 16 MPAL cases (14%) with KMT2A rearrangements, 15 of whom showed a B/myeloid immunophenotype.
- Approximately one-half of B/M MPAL cases had rearrangements of ZNF384 with recurrent fusion partners, including TCF3 and EP300. These cases had gene expression profiles indistinguishable from B-ALL cases with ZNF384 rearrangements.[
100 ] - Approximately two-thirds of B/M MPAL cases had RAS pathway alterations, with NRAS and PTPN11 being the most commonly altered genes.[
100 ] - Genes encoding epigenetic regulators (e.g., MLLT3, KDM6A, EP300, and CREBBP) are altered in approximately two-thirds of B/M MPAL cases.[
100 ]
- T/M MPAL.
- Among 115 MPAL cases for which genomic characterization was performed, 49 (43%) were T/M MPAL.[
100 ] The genomic features of the T/M MPAL cases shared commonalities with those of ETP ALL, suggesting that T/M MPAL and ETP ALL are similar entities along the spectrum of immature leukemias. - Compared with T-ALL, T/M MPAL showed a lower rate of alterations in the core T-ALL transcription factors (TAL1, TAL2, TLX1, TLX3, LMO1, LMO2, NKX2-1, HOXA10, and LYL1) (63% vs. 16%, respectively).[
100 ] A similar lower rate was also observed for ETP ALL. - CDKN2A, CDKN2B, and NOTCH1 variants, which are present in approximately two-thirds of T-ALL cases, were much less common in T/M MPAL cases. By contrast, WT1 variants occurred in approximately 40% of T/M MPAL, but in less than 10% of T-ALL cases.[
100 ] - One-third of T/M MPAL cases have genomic alterations associated with BCL11B that lead to allele-specific, generally high expression of BCL11B.[
187 ,188 ]- One such alteration is t(2;14)(q22;q32), which produces an in-frame ZEB2::BCL11B fusion gene that leads to deregulated expression of BCL11B.
- Other alterations leading to allele-specific deregulated BCL11B expression include structural variants that juxtapose regulatory sequences of active genes (e.g., ARID1B [chromosome 6], BENC [chromosome 7], and CDK6 [chromosome 7]) upstream or downstream of the BCL11B locus in a process called enhancer hijacking.
- Finally, a translocation cannot be identified in about 20% of cases with deregulated BCL11B overexpression. In such cases, amplification of a downstream enhancer, BCL11B enhancer tandem amplification (BETA), leads to BCL11B promoter driven transcription.
- There is a high prevalence of FLT3 alterations and JAK/STAT activation in acute leukemias driven by genomic alterations leading to BCL11B overexpression.
- RAS and JAK-STAT pathway variants were common in the T/M MPAL and ETP ALL cases, while the PI3K signaling pathway is more commonly altered in T-ALL.[
100 ] For T/M MPAL, the most commonly altered signaling pathway gene was FLT3 (43% of cases). FLT3 variants tended to be mutually exclusive with RAS pathway variants. - Genes encoding epigenetic regulators (e.g., EZH2 and PHF6) were altered in approximately two-thirds of T/M MPAL cases.[
100 ]
- Among 115 MPAL cases for which genomic characterization was performed, 49 (43%) were T/M MPAL.[
Gene polymorphisms in drug metabolic pathways
Several polymorphisms of genes involved in the metabolism of chemotherapeutic agents have been reported to have prognostic significance in childhood ALL.[
- TPMT.
Patients with variant phenotypes of TPMT (a gene involved in the metabolism of thiopurines such as mercaptopurine) appear to have more favorable outcomes,[
194 ] although such patients may also be at higher risk of developing significant treatment-related toxicities, including myelosuppression, infection, and second malignancies.[195 ,196 ] Patients with homozygosity for TPMT variants associated with low enzymatic activity tolerate only very low doses of mercaptopurine (approximately 10% of the standard dose) and are treated with reduced doses of mercaptopurine to avoid excessive toxicity. Patients who are heterozygous for this variant enzyme gene generally tolerate mercaptopurine without serious toxicity, but they do require more frequent dose reductions for hematologic toxicity than do patients who are homozygous for the normal allele.[197 ,198 ] - NUDT15.
Germline variants in NUDT15 that reduce or abolish activity of this enzyme also lead to diminished tolerance to thiopurines.[
197 ,199 ] The NUDT15 variants are most common in East Asian and Hispanic patients, and they are rare in European and African patients. Patients homozygous for the risk variants tolerate only very low doses of mercaptopurine, while patients heterozygous for the risk alleles tolerate lower doses than do patients homozygous for the wild-type allele (approximately 25% dose reduction on average), but there is broad overlap in tolerated doses between the two groups.[197 ,200 ] - CEP72.
Gene polymorphisms may also affect the expression of proteins that play central roles in the cellular effects of anticancer drugs. As an example, patients who are homozygous for a polymorphism in the promoter region of CEP72 (a centrosomal protein involved in microtubule formation) are at increased risk of vincristine neurotoxicity.[
201 ] - Single nucleotide polymorphisms.
Genome-wide polymorphism analysis has identified specific single nucleotide polymorphisms associated with high end-induction MRD and risk of relapse. Polymorphisms of interleukin-15, as well as genes associated with the metabolism of etoposide and methotrexate, were significantly associated with treatment response in two large cohorts of ALL patients treated on SJCRH and COG protocols.[
202 ] Polymorphic variants involving the reduced folate carrier and methotrexate metabolism have been linked to toxicity and outcome.[203 ,204 ] While these associations suggest that individual variations in drug metabolism can affect outcome, few studies have attempted to adjust for these variations. It is unknown whether individualized dose modification on the basis of these findings will improve outcomes.
References:
- Brady SW, Roberts KG, Gu Z, et al.: The genomic landscape of pediatric acute lymphoblastic leukemia. Nat Genet 54 (9): 1376-1389, 2022.
- Mullighan CG, Goorha S, Radtke I, et al.: Genome-wide analysis of genetic alterations in acute lymphoblastic leukaemia. Nature 446 (7137): 758-64, 2007.
- Mullighan CG, Miller CB, Radtke I, et al.: BCR-ABL1 lymphoblastic leukaemia is characterized by the deletion of Ikaros. Nature 453 (7191): 110-4, 2008.
- Roberts KG, Li Y, Payne-Turner D, et al.: Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med 371 (11): 1005-15, 2014.
- Lilljebjörn H, Henningsson R, Hyrenius-Wittsten A, et al.: Identification of ETV6-RUNX1-like and DUX4-rearranged subtypes in paediatric B-cell precursor acute lymphoblastic leukaemia. Nat Commun 7: 11790, 2016.
- Zhang J, McCastlain K, Yoshihara H, et al.: Deregulation of DUX4 and ERG in acute lymphoblastic leukemia. Nat Genet 48 (12): 1481-1489, 2016.
- Holmfeldt L, Wei L, Diaz-Flores E, et al.: The genomic landscape of hypodiploid acute lymphoblastic leukemia. Nat Genet 45 (3): 242-52, 2013.
- Loh ML, Zhang J, Harvey RC, et al.: Tyrosine kinome sequencing of pediatric acute lymphoblastic leukemia: a report from the Children's Oncology Group TARGET Project. Blood 121 (3): 485-8, 2013.
- Bercovich D, Ganmore I, Scott LM, et al.: Mutations of JAK2 in acute lymphoblastic leukaemias associated with Down's syndrome. Lancet 372 (9648): 1484-92, 2008.
- Li Z, Chang TC, Junco JJ, et al.: Genomic landscape of Down syndrome-associated acute lymphoblastic leukemia. Blood 142 (2): 172-184, 2023.
- Andersson AK, Ma J, Wang J, et al.: The landscape of somatic mutations in infant MLL-rearranged acute lymphoblastic leukemias. Nat Genet 47 (4): 330-7, 2015.
- Ma X, Edmonson M, Yergeau D, et al.: Rise and fall of subclones from diagnosis to relapse in pediatric B-acute lymphoblastic leukaemia. Nat Commun 6: 6604, 2015.
- Meyer JA, Wang J, Hogan LE, et al.: Relapse-specific mutations in NT5C2 in childhood acute lymphoblastic leukemia. Nat Genet 45 (3): 290-4, 2013.
- Li B, Li H, Bai Y, et al.: Negative feedback-defective PRPS1 mutants drive thiopurine resistance in relapsed childhood ALL. Nat Med 21 (6): 563-71, 2015.
- Mullighan CG, Zhang J, Kasper LH, et al.: CREBBP mutations in relapsed acute lymphoblastic leukaemia. Nature 471 (7337): 235-9, 2011.
- Mattano LA, Devidas M, Maloney KW, et al.: Favorable Trisomies and ETV6-RUNX1 Predict Cure in Low-Risk B-Cell Acute Lymphoblastic Leukemia: Results From Children's Oncology Group Trial AALL0331. J Clin Oncol 39 (14): 1540-1552, 2021.
- Moorman AV, Ensor HM, Richards SM, et al.: Prognostic effect of chromosomal abnormalities in childhood B-cell precursor acute lymphoblastic leukaemia: results from the UK Medical Research Council ALL97/99 randomised trial. Lancet Oncol 11 (5): 429-38, 2010.
- Alaggio R, Amador C, Anagnostopoulos I, et al.: The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 36 (7): 1720-1748, 2022.
- Paulsson K, Johansson B: High hyperdiploid childhood acute lymphoblastic leukemia. Genes Chromosomes Cancer 48 (8): 637-60, 2009.
- Aricò M, Valsecchi MG, Rizzari C, et al.: Long-term results of the AIEOP-ALL-95 Trial for Childhood Acute Lymphoblastic Leukemia: insight on the prognostic value of DNA index in the framework of Berlin-Frankfurt-Muenster based chemotherapy. J Clin Oncol 26 (2): 283-9, 2008.
- Dastugue N, Suciu S, Plat G, et al.: Hyperdiploidy with 58-66 chromosomes in childhood B-acute lymphoblastic leukemia is highly curable: 58951 CLG-EORTC results. Blood 121 (13): 2415-23, 2013.
- Synold TW, Relling MV, Boyett JM, et al.: Blast cell methotrexate-polyglutamate accumulation in vivo differs by lineage, ploidy, and methotrexate dose in acute lymphoblastic leukemia. J Clin Invest 94 (5): 1996-2001, 1994.
- Moorman AV, Richards SM, Martineau M, et al.: Outcome heterogeneity in childhood high-hyperdiploid acute lymphoblastic leukemia. Blood 102 (8): 2756-62, 2003.
- Chilton L, Buck G, Harrison CJ, et al.: High hyperdiploidy among adolescents and adults with acute lymphoblastic leukaemia (ALL): cytogenetic features, clinical characteristics and outcome. Leukemia 28 (7): 1511-8, 2014.
- Sutcliffe MJ, Shuster JJ, Sather HN, et al.: High concordance from independent studies by the Children's Cancer Group (CCG) and Pediatric Oncology Group (POG) associating favorable prognosis with combined trisomies 4, 10, and 17 in children with NCI Standard-Risk B-precursor Acute Lymphoblastic Leukemia: a Children's Oncology Group (COG) initiative. Leukemia 19 (5): 734-40, 2005.
- Harris MB, Shuster JJ, Carroll A, et al.: Trisomy of leukemic cell chromosomes 4 and 10 identifies children with B-progenitor cell acute lymphoblastic leukemia with a very low risk of treatment failure: a Pediatric Oncology Group study. Blood 79 (12): 3316-24, 1992.
- Enshaei A, Vora A, Harrison CJ, et al.: Defining low-risk high hyperdiploidy in patients with paediatric acute lymphoblastic leukaemia: a retrospective analysis of data from the UKALL97/99 and UKALL2003 clinical trials. Lancet Haematol 8 (11): e828-e839, 2021.
- Heerema NA, Harbott J, Galimberti S, et al.: Secondary cytogenetic aberrations in childhood Philadelphia chromosome positive acute lymphoblastic leukemia are nonrandom and may be associated with outcome. Leukemia 18 (4): 693-702, 2004.
- Carroll AJ, Shago M, Mikhail FM, et al.: Masked hypodiploidy: Hypodiploid acute lymphoblastic leukemia (ALL) mimicking hyperdiploid ALL in children: A report from the Children's Oncology Group. Cancer Genet 238: 62-68, 2019.
- Nachman JB, Heerema NA, Sather H, et al.: Outcome of treatment in children with hypodiploid acute lymphoblastic leukemia. Blood 110 (4): 1112-5, 2007.
- Raimondi SC, Zhou Y, Shurtleff SA, et al.: Near-triploidy and near-tetraploidy in childhood acute lymphoblastic leukemia: association with B-lineage blast cells carrying the ETV6-RUNX1 fusion, T-lineage immunophenotype, and favorable outcome. Cancer Genet Cytogenet 169 (1): 50-7, 2006.
- Attarbaschi A, Mann G, König M, et al.: Incidence and relevance of secondary chromosome abnormalities in childhood TEL/AML1+ acute lymphoblastic leukemia: an interphase FISH analysis. Leukemia 18 (10): 1611-6, 2004.
- Lemez P, Attarbaschi A, Béné MC, et al.: Childhood near-tetraploid acute lymphoblastic leukemia: an EGIL study on 36 cases. Eur J Haematol 85 (4): 300-8, 2010.
- Paulsson K, Lilljebjörn H, Biloglav A, et al.: The genomic landscape of high hyperdiploid childhood acute lymphoblastic leukemia. Nat Genet 47 (6): 672-6, 2015.
- Harrison CJ, Moorman AV, Broadfield ZJ, et al.: Three distinct subgroups of hypodiploidy in acute lymphoblastic leukaemia. Br J Haematol 125 (5): 552-9, 2004.
- Mullighan CG, Jeha S, Pei D, et al.: Outcome of children with hypodiploid ALL treated with risk-directed therapy based on MRD levels. Blood 126 (26): 2896-9, 2015.
- Pui CH, Rebora P, Schrappe M, et al.: Outcome of Children With Hypodiploid Acute Lymphoblastic Leukemia: A Retrospective Multinational Study. J Clin Oncol 37 (10): 770-779, 2019.
- McNeer JL, Devidas M, Dai Y, et al.: Hematopoietic Stem-Cell Transplantation Does Not Improve the Poor Outcome of Children With Hypodiploid Acute Lymphoblastic Leukemia: A Report From Children's Oncology Group. J Clin Oncol 37 (10): 780-789, 2019.
- Irving J, Matheson E, Minto L, et al.: Ras pathway mutations are prevalent in relapsed childhood acute lymphoblastic leukemia and confer sensitivity to MEK inhibition. Blood 124 (23): 3420-30, 2014.
- Qian M, Cao X, Devidas M, et al.: TP53 Germline Variations Influence the Predisposition and Prognosis of B-Cell Acute Lymphoblastic Leukemia in Children. J Clin Oncol 36 (6): 591-599, 2018.
- Rubnitz JE, Wichlan D, Devidas M, et al.: Prospective analysis of TEL gene rearrangements in childhood acute lymphoblastic leukemia: a Children's Oncology Group study. J Clin Oncol 26 (13): 2186-91, 2008.
- Kanerva J, Saarinen-Pihkala UM, Niini T, et al.: Favorable outcome in 20-year follow-up of children with very-low-risk ALL and minimal standard therapy, with special reference to TEL-AML1 fusion. Pediatr Blood Cancer 42 (1): 30-5, 2004.
- Aldrich MC, Zhang L, Wiemels JL, et al.: Cytogenetics of Hispanic and White children with acute lymphoblastic leukemia in California. Cancer Epidemiol Biomarkers Prev 15 (3): 578-81, 2006.
- Loh ML, Goldwasser MA, Silverman LB, et al.: Prospective analysis of TEL/AML1-positive patients treated on Dana-Farber Cancer Institute Consortium Protocol 95-01. Blood 107 (11): 4508-13, 2006.
- Borowitz MJ, Devidas M, Hunger SP, et al.: Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children's Oncology Group study. Blood 111 (12): 5477-85, 2008.
- Madzo J, Zuna J, Muzíková K, et al.: Slower molecular response to treatment predicts poor outcome in patients with TEL/AML1 positive acute lymphoblastic leukemia: prospective real-time quantitative reverse transcriptase-polymerase chain reaction study. Cancer 97 (1): 105-13, 2003.
- Bhojwani D, Pei D, Sandlund JT, et al.: ETV6-RUNX1-positive childhood acute lymphoblastic leukemia: improved outcome with contemporary therapy. Leukemia 26 (2): 265-70, 2012.
- Enshaei A, Schwab CJ, Konn ZJ, et al.: Long-term follow-up of ETV6-RUNX1 ALL reveals that NCI risk, rather than secondary genetic abnormalities, is the key risk factor. Leukemia 27 (11): 2256-9, 2013.
- Barbany G, Andersen MK, Autio K, et al.: Additional aberrations of the ETV6 and RUNX1 genes have no prognostic impact in 229 t(12;21)(p13;q22)-positive B-cell precursor acute lymphoblastic leukaemias treated according to the NOPHO-ALL-2000 protocol. Leuk Res 36 (7): 936-8, 2012.
- Forestier E, Heyman M, Andersen MK, et al.: Outcome of ETV6/RUNX1-positive childhood acute lymphoblastic leukaemia in the NOPHO-ALL-1992 protocol: frequent late relapses but good overall survival. Br J Haematol 140 (6): 665-72, 2008.
- Seeger K, Stackelberg AV, Taube T, et al.: Relapse of TEL-AML1--positive acute lymphoblastic leukemia in childhood: a matched-pair analysis. J Clin Oncol 19 (13): 3188-93, 2001.
- Gandemer V, Chevret S, Petit A, et al.: Excellent prognosis of late relapses of ETV6/RUNX1-positive childhood acute lymphoblastic leukemia: lessons from the FRALLE 93 protocol. Haematologica 97 (11): 1743-50, 2012.
- Zuna J, Ford AM, Peham M, et al.: TEL deletion analysis supports a novel view of relapse in childhood acute lymphoblastic leukemia. Clin Cancer Res 10 (16): 5355-60, 2004.
- van Delft FW, Horsley S, Colman S, et al.: Clonal origins of relapse in ETV6-RUNX1 acute lymphoblastic leukemia. Blood 117 (23): 6247-54, 2011.
- Aricò M, Schrappe M, Hunger SP, et al.: Clinical outcome of children with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia treated between 1995 and 2005. J Clin Oncol 28 (31): 4755-61, 2010.
- Schrappe M, Aricò M, Harbott J, et al.: Philadelphia chromosome-positive (Ph+) childhood acute lymphoblastic leukemia: good initial steroid response allows early prediction of a favorable treatment outcome. Blood 92 (8): 2730-41, 1998.
- Ribeiro RC, Broniscer A, Rivera GK, et al.: Philadelphia chromosome-positive acute lymphoblastic leukemia in children: durable responses to chemotherapy associated with low initial white blood cell counts. Leukemia 11 (9): 1493-6, 1997.
- Biondi A, Schrappe M, De Lorenzo P, et al.: Imatinib after induction for treatment of children and adolescents with Philadelphia-chromosome-positive acute lymphoblastic leukaemia (EsPhALL): a randomised, open-label, intergroup study. Lancet Oncol 13 (9): 936-45, 2012.
- Schultz KR, Bowman WP, Aledo A, et al.: Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: a children's oncology group study. J Clin Oncol 27 (31): 5175-81, 2009.
- Schultz KR, Carroll A, Heerema NA, et al.: Long-term follow-up of imatinib in pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia: Children's Oncology Group study AALL0031. Leukemia 28 (7): 1467-71, 2014.
- Duffield AS, Mullighan CG, Borowitz MJ: International Consensus Classification of acute lymphoblastic leukemia/lymphoma. Virchows Arch 482 (1): 11-26, 2023.
- Short NJ, Jabbour E, Macaron W, et al.: Ultrasensitive NGS MRD assessment in Ph+ ALL: Prognostic impact and correlation with RT-PCR for BCR::ABL1. Am J Hematol 98 (8): 1196-1203, 2023.
- Hovorkova L, Zaliova M, Venn NC, et al.: Monitoring of childhood ALL using BCR-ABL1 genomic breakpoints identifies a subgroup with CML-like biology. Blood 129 (20): 2771-2781, 2017.
- Zuna J, Hovorkova L, Krotka J, et al.: Minimal residual disease in BCR::ABL1-positive acute lymphoblastic leukemia: different significance in typical ALL and in CML-like disease. Leukemia 36 (12): 2793-2801, 2022.
- Hunger SP, Tran TH, Saha V, et al.: Dasatinib with intensive chemotherapy in de novo paediatric Philadelphia chromosome-positive acute lymphoblastic leukaemia (CA180-372/COG AALL1122): a single-arm, multicentre, phase 2 trial. Lancet Haematol 10 (7): e510-e520, 2023.
- Pui CH, Chessells JM, Camitta B, et al.: Clinical heterogeneity in childhood acute lymphoblastic leukemia with 11q23 rearrangements. Leukemia 17 (4): 700-6, 2003.
- Johansson B, Moorman AV, Haas OA, et al.: Hematologic malignancies with t(4;11)(q21;q23)--a cytogenetic, morphologic, immunophenotypic and clinical study of 183 cases. European 11q23 Workshop participants. Leukemia 12 (5): 779-87, 1998.
- Raimondi SC, Peiper SC, Kitchingman GR, et al.: Childhood acute lymphoblastic leukemia with chromosomal breakpoints at 11q23. Blood 73 (6): 1627-34, 1989.
- Harrison CJ, Moorman AV, Barber KE, et al.: Interphase molecular cytogenetic screening for chromosomal abnormalities of prognostic significance in childhood acute lymphoblastic leukaemia: a UK Cancer Cytogenetics Group Study. Br J Haematol 129 (4): 520-30, 2005.
- Pui CH, Pei D, Campana D, et al.: A revised definition for cure of childhood acute lymphoblastic leukemia. Leukemia 28 (12): 2336-43, 2014.
- Pieters R, Schrappe M, De Lorenzo P, et al.: A treatment protocol for infants younger than 1 year with acute lymphoblastic leukaemia (Interfant-99): an observational study and a multicentre randomised trial. Lancet 370 (9583): 240-50, 2007.
- Attarbaschi A, Möricke A, Harrison CJ, et al.: Outcomes of Childhood Noninfant Acute Lymphoblastic Leukemia With 11q23/KMT2A Rearrangements in a Modern Therapy Era: A Retrospective International Study. J Clin Oncol 41 (7): 1404-1422, 2023.
- Isobe T, Takagi M, Sato-Otsubo A, et al.: Multi-omics analysis defines highly refractory RAS burdened immature subgroup of infant acute lymphoblastic leukemia. Nat Commun 13 (1): 4501, 2022.
- Pui CH, Gaynon PS, Boyett JM, et al.: Outcome of treatment in childhood acute lymphoblastic leukaemia with rearrangements of the 11q23 chromosomal region. Lancet 359 (9321): 1909-15, 2002.
- Rubnitz JE, Camitta BM, Mahmoud H, et al.: Childhood acute lymphoblastic leukemia with the MLL-ENL fusion and t(11;19)(q23;p13.3) translocation. J Clin Oncol 17 (1): 191-6, 1999.
- Hunger SP: Chromosomal translocations involving the E2A gene in acute lymphoblastic leukemia: clinical features and molecular pathogenesis. Blood 87 (4): 1211-24, 1996.
- Uckun FM, Sensel MG, Sather HN, et al.: Clinical significance of translocation t(1;19) in childhood acute lymphoblastic leukemia in the context of contemporary therapies: a report from the Children's Cancer Group. J Clin Oncol 16 (2): 527-35, 1998.
- Fischer U, Forster M, Rinaldi A, et al.: Genomics and drug profiling of fatal TCF3-HLF-positive acute lymphoblastic leukemia identifies recurrent mutation patterns and therapeutic options. Nat Genet 47 (9): 1020-9, 2015.
- Pui CH, Sandlund JT, Pei D, et al.: Results of therapy for acute lymphoblastic leukemia in black and white children. JAMA 290 (15): 2001-7, 2003.
- Crist WM, Carroll AJ, Shuster JJ, et al.: Poor prognosis of children with pre-B acute lymphoblastic leukemia is associated with the t(1;19)(q23;p13): a Pediatric Oncology Group study. Blood 76 (1): 117-22, 1990.
- Andersen MK, Autio K, Barbany G, et al.: Paediatric B-cell precursor acute lymphoblastic leukaemia with t(1;19)(q23;p13): clinical and cytogenetic characteristics of 47 cases from the Nordic countries treated according to NOPHO protocols. Br J Haematol 155 (2): 235-43, 2011.
- Pui CH, Campana D, Pei D, et al.: Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med 360 (26): 2730-41, 2009.
- Jeha S, Pei D, Raimondi SC, et al.: Increased risk for CNS relapse in pre-B cell leukemia with the t(1;19)/TCF3-PBX1. Leukemia 23 (8): 1406-9, 2009.
- Minson KA, Prasad P, Vear S, et al.: t(17;19) in Children with Acute Lymphocytic Leukemia: A Report of 3 Cases and a Review of the Literature. Case Rep Hematol 2013: 563291, 2013.
- Lee SHR, Antillon-Klussmann F, Pei D, et al.: Association of Genetic Ancestry With the Molecular Subtypes and Prognosis of Childhood Acute Lymphoblastic Leukemia. JAMA Oncol 8 (3): 354-363, 2022.
- Zaliova M, Potuckova E, Hovorkova L, et al.: ERG deletions in childhood acute lymphoblastic leukemia with DUX4 rearrangements are mostly polyclonal, prognostically relevant and their detection rate strongly depends on screening method sensitivity. Haematologica 104 (7): 1407-1416, 2019.
- Harvey RC, Mullighan CG, Wang X, et al.: Identification of novel cluster groups in pediatric high-risk B-precursor acute lymphoblastic leukemia with gene expression profiling: correlation with genome-wide DNA copy number alterations, clinical characteristics, and outcome. Blood 116 (23): 4874-84, 2010.
- Zaliova M, Zimmermannova O, Dörge P, et al.: ERG deletion is associated with CD2 and attenuates the negative impact of IKZF1 deletion in childhood acute lymphoblastic leukemia. Leukemia 28 (1): 182-5, 2014.
- Clappier E, Auclerc MF, Rapion J, et al.: An intragenic ERG deletion is a marker of an oncogenic subtype of B-cell precursor acute lymphoblastic leukemia with a favorable outcome despite frequent IKZF1 deletions. Leukemia 28 (1): 70-7, 2014.
- Li Z, Lee SHR, Chin WHN, et al.: Distinct clinical characteristics of DUX4- and PAX5-altered childhood B-lymphoblastic leukemia. Blood Adv 5 (23): 5226-5238, 2021.
- Gu Z, Churchman M, Roberts K, et al.: Genomic analyses identify recurrent MEF2D fusions in acute lymphoblastic leukaemia. Nat Commun 7: 13331, 2016.
- Liu YF, Wang BY, Zhang WN, et al.: Genomic Profiling of Adult and Pediatric B-cell Acute Lymphoblastic Leukemia. EBioMedicine 8: 173-83, 2016.
- Suzuki K, Okuno Y, Kawashima N, et al.: MEF2D-BCL9 Fusion Gene Is Associated With High-Risk Acute B-Cell Precursor Lymphoblastic Leukemia in Adolescents. J Clin Oncol 34 (28): 3451-9, 2016.
- Lilljebjörn H, Ågerstam H, Orsmark-Pietras C, et al.: RNA-seq identifies clinically relevant fusion genes in leukemia including a novel MEF2D/CSF1R fusion responsive to imatinib. Leukemia 28 (4): 977-9, 2014.
- Hirabayashi S, Ohki K, Nakabayashi K, et al.: ZNF384-related fusion genes define a subgroup of childhood B-cell precursor acute lymphoblastic leukemia with a characteristic immunotype. Haematologica 102 (1): 118-129, 2017.
- Qian M, Zhang H, Kham SK, et al.: Whole-transcriptome sequencing identifies a distinct subtype of acute lymphoblastic leukemia with predominant genomic abnormalities of EP300 and CREBBP. Genome Res 27 (2): 185-195, 2017.
- Hirabayashi S, Butler ER, Ohki K, et al.: Clinical characteristics and outcomes of B-ALL with ZNF384 rearrangements: a retrospective analysis by the Ponte di Legno Childhood ALL Working Group. Leukemia 35 (11): 3272-3277, 2021.
- Shago M, Abla O, Hitzler J, et al.: Frequency and outcome of pediatric acute lymphoblastic leukemia with ZNF384 gene rearrangements including a novel translocation resulting in an ARID1B/ZNF384 gene fusion. Pediatr Blood Cancer 63 (11): 1915-21, 2016.
- Yao L, Cen J, Pan J, et al.: TAF15-ZNF384 fusion gene in childhood mixed phenotype acute leukemia. Cancer Genet 211: 1-4, 2017.
- Alexander TB, Gu Z, Iacobucci I, et al.: The genetic basis and cell of origin of mixed phenotype acute leukaemia. Nature 562 (7727): 373-379, 2018.
- Boer JM, Valsecchi MG, Hormann FM, et al.: Favorable outcome of NUTM1-rearranged infant and pediatric B cell precursor acute lymphoblastic leukemia in a collaborative international study. Leukemia 35 (10): 2978-2982, 2021.
- De Lorenzo P, Moorman AV, Pieters R, et al.: Cytogenetics and outcome of infants with acute lymphoblastic leukemia and absence of MLL rearrangements. Leukemia 28 (2): 428-30, 2014.
- Fazio G, Bardini M, De Lorenzo P, et al.: Recurrent genetic fusions redefine MLL germ line acute lymphoblastic leukemia in infants. Blood 137 (14): 1980-1984, 2021.
- Arber DA, Orazi A, Hasserjian R, et al.: The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127 (20): 2391-405, 2016.
- Hogan TF, Koss W, Murgo AJ, et al.: Acute lymphoblastic leukemia with chromosomal 5;14 translocation and hypereosinophilia: case report and literature review. J Clin Oncol 5 (3): 382-90, 1987.
- Grimaldi JC, Meeker TC: The t(5;14) chromosomal translocation in a case of acute lymphocytic leukemia joins the interleukin-3 gene to the immunoglobulin heavy chain gene. Blood 73 (8): 2081-5, 1989.
- Meeker TC, Hardy D, Willman C, et al.: Activation of the interleukin-3 gene by chromosome translocation in acute lymphocytic leukemia with eosinophilia. Blood 76 (2): 285-9, 1990.
- Sutton R, Lonergan M, Tapp H, et al.: Two cases of hypereosinophilia and high-risk acute lymphoblastic leukemia. Leukemia 22 (7): 1463-5, 2008.
- Koleilat A, Smadbeck JB, Zepeda-Mendoza CJ, et al.: Characterization of unusual iAMP21 B-lymphoblastic leukemia (iAMP21-ALL) from the Mayo Clinic and Children's Oncology Group. Genes Chromosomes Cancer 61 (12): 710-719, 2022.
- Heerema NA, Carroll AJ, Devidas M, et al.: Intrachromosomal amplification of chromosome 21 is associated with inferior outcomes in children with acute lymphoblastic leukemia treated in contemporary standard-risk children's oncology group studies: a report from the children's oncology group. J Clin Oncol 31 (27): 3397-402, 2013.
- Moorman AV, Robinson H, Schwab C, et al.: Risk-directed treatment intensification significantly reduces the risk of relapse among children and adolescents with acute lymphoblastic leukemia and intrachromosomal amplification of chromosome 21: a comparison of the MRC ALL97/99 and UKALL2003 trials. J Clin Oncol 31 (27): 3389-96, 2013.
- Harrison CJ, Moorman AV, Schwab C, et al.: An international study of intrachromosomal amplification of chromosome 21 (iAMP21): cytogenetic characterization and outcome. Leukemia 28 (5): 1015-21, 2014.
- Gu Z, Churchman ML, Roberts KG, et al.: PAX5-driven subtypes of B-progenitor acute lymphoblastic leukemia. Nat Genet 51 (2): 296-307, 2019.
- Strehl S, König M, Dworzak MN, et al.: PAX5/ETV6 fusion defines cytogenetic entity dic(9;12)(p13;p13). Leukemia 17 (6): 1121-3, 2003.
- Schwab C, Nebral K, Chilton L, et al.: Intragenic amplification of PAX5: a novel subgroup in B-cell precursor acute lymphoblastic leukemia? Blood Adv 1 (19): 1473-7, 2017.
- Den Boer ML, van Slegtenhorst M, De Menezes RX, et al.: A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: a genome-wide classification study. Lancet Oncol 10 (2): 125-34, 2009.
- Mullighan CG, Su X, Zhang J, et al.: Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N Engl J Med 360 (5): 470-80, 2009.
- Reshmi SC, Harvey RC, Roberts KG, et al.: Targetable kinase gene fusions in high-risk B-ALL: a study from the Children's Oncology Group. Blood 129 (25): 3352-3361, 2017.
- Roberts KG, Morin RD, Zhang J, et al.: Genetic alterations activating kinase and cytokine receptor signaling in high-risk acute lymphoblastic leukemia. Cancer Cell 22 (2): 153-66, 2012.
- van der Veer A, Waanders E, Pieters R, et al.: Independent prognostic value of BCR-ABL1-like signature and IKZF1 deletion, but not high CRLF2 expression, in children with B-cell precursor ALL. Blood 122 (15): 2622-9, 2013.
- Roberts KG, Reshmi SC, Harvey RC, et al.: Genomic and outcome analyses of Ph-like ALL in NCI standard-risk patients: a report from the Children's Oncology Group. Blood 132 (8): 815-824, 2018.
- Roberts KG, Pei D, Campana D, et al.: Outcomes of children with BCR-ABL1–like acute lymphoblastic leukemia treated with risk-directed therapy based on the levels of minimal residual disease. J Clin Oncol 32 (27): 3012-20, 2014.
- Harvey RC, Mullighan CG, Chen IM, et al.: Rearrangement of CRLF2 is associated with mutation of JAK kinases, alteration of IKZF1, Hispanic/Latino ethnicity, and a poor outcome in pediatric B-progenitor acute lymphoblastic leukemia. Blood 115 (26): 5312-21, 2010.
- Mullighan CG, Collins-Underwood JR, Phillips LA, et al.: Rearrangement of CRLF2 in B-progenitor- and Down syndrome-associated acute lymphoblastic leukemia. Nat Genet 41 (11): 1243-6, 2009.
- Schwab C, Roberts K, Boer JM, et al.: SSBP2-CSF1R is a recurrent fusion in B-lineage acute lymphoblastic leukemia with diverse genetic presentation and variable outcome. Blood 137 (13): 1835-1838, 2021.
- den Boer ML, Cario G, Moorman AV, et al.: Outcomes of paediatric patients with B-cell acute lymphocytic leukaemia with ABL-class fusion in the pre-tyrosine-kinase inhibitor era: a multicentre, retrospective, cohort study. Lancet Haematol 8 (1): e55-e66, 2021.
- Iacobucci I, Li Y, Roberts KG, et al.: Truncating Erythropoietin Receptor Rearrangements in Acute Lymphoblastic Leukemia. Cancer Cell 29 (2): 186-200, 2016.
- Cario G, Zimmermann M, Romey R, et al.: Presence of the P2RY8-CRLF2 rearrangement is associated with a poor prognosis in non-high-risk precursor B-cell acute lymphoblastic leukemia in children treated according to the ALL-BFM 2000 protocol. Blood 115 (26): 5393-7, 2010.
- Ensor HM, Schwab C, Russell LJ, et al.: Demographic, clinical, and outcome features of children with acute lymphoblastic leukemia and CRLF2 deregulation: results from the MRC ALL97 clinical trial. Blood 117 (7): 2129-36, 2011.
- Schmäh J, Fedders B, Panzer-Grümayer R, et al.: Molecular characterization of acute lymphoblastic leukemia with high CRLF2 gene expression in childhood. Pediatr Blood Cancer 64 (10): , 2017.
- Raca G, Abdel-Azim H, Yue F, et al.: Increased Incidence of IKZF1 deletions and IGH-CRLF2 translocations in B-ALL of Hispanic/Latino children-a novel health disparity. Leukemia 35 (8): 2399-2402, 2021.
- Vesely C, Frech C, Eckert C, et al.: Genomic and transcriptional landscape of P2RY8-CRLF2-positive childhood acute lymphoblastic leukemia. Leukemia 31 (7): 1491-1501, 2017.
- Russell LJ, Jones L, Enshaei A, et al.: Characterisation of the genomic landscape of CRLF2-rearranged acute lymphoblastic leukemia. Genes Chromosomes Cancer 56 (5): 363-372, 2017.
- Potter N, Jones L, Blair H, et al.: Single-cell analysis identifies CRLF2 rearrangements as both early and late events in Down syndrome and non-Down syndrome acute lymphoblastic leukaemia. Leukemia 33 (4): 893-904, 2019.
- Morak M, Attarbaschi A, Fischer S, et al.: Small sizes and indolent evolutionary dynamics challenge the potential role of P2RY8-CRLF2-harboring clones as main relapse-driving force in childhood ALL. Blood 120 (26): 5134-42, 2012.
- Schwab CJ, Chilton L, Morrison H, et al.: Genes commonly deleted in childhood B-cell precursor acute lymphoblastic leukemia: association with cytogenetics and clinical features. Haematologica 98 (7): 1081-8, 2013.
- Chen IM, Harvey RC, Mullighan CG, et al.: Outcome modeling with CRLF2, IKZF1, JAK, and minimal residual disease in pediatric acute lymphoblastic leukemia: a Children's Oncology Group study. Blood 119 (15): 3512-22, 2012.
- Palmi C, Vendramini E, Silvestri D, et al.: Poor prognosis for P2RY8-CRLF2 fusion but not for CRLF2 over-expression in children with intermediate risk B-cell precursor acute lymphoblastic leukemia. Leukemia 26 (10): 2245-53, 2012.
- Clappier E, Grardel N, Bakkus M, et al.: IKZF1 deletion is an independent prognostic marker in childhood B-cell precursor acute lymphoblastic leukemia, and distinguishes patients benefiting from pulses during maintenance therapy: results of the EORTC Children's Leukemia Group study 58951. Leukemia 29 (11): 2154-61, 2015.
- Srinivasan S, Ramanathan S, Kumar S, et al.: Prevalence and prognostic significance of IKZF1 deletion in paediatric acute lymphoblastic leukemia: A systematic review and meta-analysis. Ann Hematol 102 (8): 2165-2179, 2023.
- Buitenkamp TD, Pieters R, Gallimore NE, et al.: Outcome in children with Down's syndrome and acute lymphoblastic leukemia: role of IKZF1 deletions and CRLF2 aberrations. Leukemia 26 (10): 2204-11, 2012.
- Krentz S, Hof J, Mendioroz A, et al.: Prognostic value of genetic alterations in children with first bone marrow relapse of childhood B-cell precursor acute lymphoblastic leukemia. Leukemia 27 (2): 295-304, 2013.
- Feng J, Tang Y: Prognostic significance of IKZF1 alteration status in pediatric B-lineage acute lymphoblastic leukemia: a meta-analysis. Leuk Lymphoma 54 (4): 889-91, 2013.
- Dörge P, Meissner B, Zimmermann M, et al.: IKZF1 deletion is an independent predictor of outcome in pediatric acute lymphoblastic leukemia treated according to the ALL-BFM 2000 protocol. Haematologica 98 (3): 428-32, 2013.
- Olsson L, Castor A, Behrendtz M, et al.: Deletions of IKZF1 and SPRED1 are associated with poor prognosis in a population-based series of pediatric B-cell precursor acute lymphoblastic leukemia diagnosed between 1992 and 2011. Leukemia 28 (2): 302-10, 2014.
- Boer JM, van der Veer A, Rizopoulos D, et al.: Prognostic value of rare IKZF1 deletion in childhood B-cell precursor acute lymphoblastic leukemia: an international collaborative study. Leukemia 30 (1): 32-8, 2016.
- Tran TH, Harris MH, Nguyen JV, et al.: Prognostic impact of kinase-activating fusions and IKZF1 deletions in pediatric high-risk B-lineage acute lymphoblastic leukemia. Blood Adv 2 (5): 529-533, 2018.
- Vrooman LM, Blonquist TM, Harris MH, et al.: Refining risk classification in childhood B acute lymphoblastic leukemia: results of DFCI ALL Consortium Protocol 05-001. Blood Adv 2 (12): 1449-1458, 2018.
- Öfverholm I, Rezayee F, Heyman M, et al.: The prognostic impact of IKZF1 deletions and UKALL genetic classifiers in paediatric B-cell precursor acute lymphoblastic leukaemia treated according to NOPHO 2008 protocols. Br J Haematol 202 (2): 384-392, 2023.
- van der Veer A, Zaliova M, Mottadelli F, et al.: IKZF1 status as a prognostic feature in BCR-ABL1-positive childhood ALL. Blood 123 (11): 1691-8, 2014.
- Stanulla M, Dagdan E, Zaliova M, et al.: IKZF1plus Defines a New Minimal Residual Disease-Dependent Very-Poor Prognostic Profile in Pediatric B-Cell Precursor Acute Lymphoblastic Leukemia. J Clin Oncol 36 (12): 1240-1249, 2018.
- Mangum DS, Meyer JA, Mason CC, et al.: Association of Combined Focal 22q11.22 Deletion and IKZF1 Alterations With Outcomes in Childhood Acute Lymphoblastic Leukemia. JAMA Oncol 7 (10): 1521-1528, 2021.
- Yeoh AEJ, Lu Y, Chin WHN, et al.: Intensifying Treatment of Childhood B-Lymphoblastic Leukemia With IKZF1 Deletion Reduces Relapse and Improves Overall Survival: Results of Malaysia-Singapore ALL 2010 Study. J Clin Oncol 36 (26): 2726-2735, 2018.
- Pieters R, de Groot-Kruseman H, Fiocco M, et al.: Improved Outcome for ALL by Prolonging Therapy for IKZF1 Deletion and Decreasing Therapy for Other Risk Groups. J Clin Oncol 41 (25): 4130-4142, 2023.
- Herbrueggen H, Mueller S, Rohde J, et al.: Treatment and outcome of IG-MYC+ neoplasms with precursor B-cell phenotype in childhood and adolescence. Leukemia 34 (3): 942-946, 2020.
- Sakaguchi K, Imamura T, Ishimaru S, et al.: Nationwide study of pediatric B-cell precursor acute lymphoblastic leukemia with chromosome 8q24/MYC rearrangement in Japan. Pediatr Blood Cancer 67 (7): e28341, 2020.
- Bomken S, Enshaei A, Schwalbe EC, et al.: Molecular characterization and clinical outcome of B-cell precursor acute lymphoblastic leukemia with IG-MYC rearrangement. Haematologica 108 (3): 717-731, 2023.
- Liu Y, Easton J, Shao Y, et al.: The genomic landscape of pediatric and young adult T-lineage acute lymphoblastic leukemia. Nat Genet 49 (8): 1211-1218, 2017.
- Armstrong SA, Look AT: Molecular genetics of acute lymphoblastic leukemia. J Clin Oncol 23 (26): 6306-15, 2005.
- Karrman K, Forestier E, Heyman M, et al.: Clinical and cytogenetic features of a population-based consecutive series of 285 pediatric T-cell acute lymphoblastic leukemias: rare T-cell receptor gene rearrangements are associated with poor outcome. Genes Chromosomes Cancer 48 (9): 795-805, 2009.
- Mansour MR, Abraham BJ, Anders L, et al.: Oncogene regulation. An oncogenic super-enhancer formed through somatic mutation of a noncoding intergenic element. Science 346 (6215): 1373-7, 2014.
- Steimlé T, Dourthe ME, Alcantara M, et al.: Clinico-biological features of T-cell acute lymphoblastic leukemia with fusion proteins. Blood Cancer J 12 (1): 14, 2022.
- Weng AP, Ferrando AA, Lee W, et al.: Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science 306 (5694): 269-71, 2004.
- Gallo Llorente L, Luther H, Schneppenheim R, et al.: Identification of novel NOTCH1 mutations: increasing our knowledge of the NOTCH signaling pathway. Pediatr Blood Cancer 61 (5): 788-96, 2014.
- Burns MA, Place AE, Stevenson KE, et al.: Identification of prognostic factors in childhood T-cell acute lymphoblastic leukemia: Results from DFCI ALL Consortium Protocols 05-001 and 11-001. Pediatr Blood Cancer 68 (1): e28719, 2021.
- Petit A, Trinquand A, Chevret S, et al.: Oncogenetic mutations combined with MRD improve outcome prediction in pediatric T-cell acute lymphoblastic leukemia. Blood 131 (3): 289-300, 2018.
- Trinquand A, Tanguy-Schmidt A, Ben Abdelali R, et al.: Toward a NOTCH1/FBXW7/RAS/PTEN-based oncogenetic risk classification of adult T-cell acute lymphoblastic leukemia: a Group for Research in Adult Acute Lymphoblastic Leukemia study. J Clin Oncol 31 (34): 4333-42, 2013.
- Bergeron J, Clappier E, Radford I, et al.: Prognostic and oncogenic relevance of TLX1/HOX11 expression level in T-ALLs. Blood 110 (7): 2324-30, 2007.
- van Grotel M, Meijerink JP, Beverloo HB, et al.: The outcome of molecular-cytogenetic subgroups in pediatric T-cell acute lymphoblastic leukemia: a retrospective study of patients treated according to DCOG or COALL protocols. Haematologica 91 (9): 1212-21, 2006.
- Cavé H, Suciu S, Preudhomme C, et al.: Clinical significance of HOX11L2 expression linked to t(5;14)(q35;q32), of HOX11 expression, and of SIL-TAL fusion in childhood T-cell malignancies: results of EORTC studies 58881 and 58951. Blood 103 (2): 442-50, 2004.
- Baak U, Gökbuget N, Orawa H, et al.: Thymic adult T-cell acute lymphoblastic leukemia stratified in standard- and high-risk group by aberrant HOX11L2 expression: experience of the German multicenter ALL study group. Leukemia 22 (6): 1154-60, 2008.
- Ferrando AA, Neuberg DS, Dodge RK, et al.: Prognostic importance of TLX1 (HOX11) oncogene expression in adults with T-cell acute lymphoblastic leukaemia. Lancet 363 (9408): 535-6, 2004.
- Burmeister T, Gökbuget N, Reinhardt R, et al.: NUP214-ABL1 in adult T-ALL: the GMALL study group experience. Blood 108 (10): 3556-9, 2006.
- Graux C, Stevens-Kroef M, Lafage M, et al.: Heterogeneous patterns of amplification of the NUP214-ABL1 fusion gene in T-cell acute lymphoblastic leukemia. Leukemia 23 (1): 125-33, 2009.
- Hagemeijer A, Graux C: ABL1 rearrangements in T-cell acute lymphoblastic leukemia. Genes Chromosomes Cancer 49 (4): 299-308, 2010.
- Quintás-Cardama A, Tong W, Manshouri T, et al.: Activity of tyrosine kinase inhibitors against human NUP214-ABL1-positive T cell malignancies. Leukemia 22 (6): 1117-24, 2008.
- Clarke S, O'Reilly J, Romeo G, et al.: NUP214-ABL1 positive T-cell acute lymphoblastic leukemia patient shows an initial favorable response to imatinib therapy post relapse. Leuk Res 35 (7): e131-3, 2011.
- Deenik W, Beverloo HB, van der Poel-van de Luytgaarde SC, et al.: Rapid complete cytogenetic remission after upfront dasatinib monotherapy in a patient with a NUP214-ABL1-positive T-cell acute lymphoblastic leukemia. Leukemia 23 (3): 627-9, 2009.
- Crombet O, Lastrapes K, Zieske A, et al.: Complete morphologic and molecular remission after introduction of dasatinib in the treatment of a pediatric patient with t-cell acute lymphoblastic leukemia and ABL1 amplification. Pediatr Blood Cancer 59 (2): 333-4, 2012.
- Seki M, Kimura S, Isobe T, et al.: Recurrent SPI1 (PU.1) fusions in high-risk pediatric T cell acute lymphoblastic leukemia. Nat Genet 49 (8): 1274-1281, 2017.
- Nagel S, Scherr M, Kel A, et al.: Activation of TLX3 and NKX2-5 in t(5;14)(q35;q32) T-cell acute lymphoblastic leukemia by remote 3'-BCL11B enhancers and coregulation by PU.1 and HMGA1. Cancer Res 67 (4): 1461-71, 2007.
- Gutierrez A, Kentsis A, Sanda T, et al.: The BCL11B tumor suppressor is mutated across the major molecular subtypes of T-cell acute lymphoblastic leukemia. Blood 118 (15): 4169-73, 2011.
- Ceppi F, Gotti G, Möricke A, et al.: Near-tetraploid T-cell acute lymphoblastic leukaemia in childhood: Results of the AIEOP-BFM ALL studies. Eur J Cancer 175: 120-124, 2022.
- Zhang J, Ding L, Holmfeldt L, et al.: The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature 481 (7380): 157-63, 2012.
- Gutierrez A, Dahlberg SE, Neuberg DS, et al.: Absence of biallelic TCRgamma deletion predicts early treatment failure in pediatric T-cell acute lymphoblastic leukemia. J Clin Oncol 28 (24): 3816-23, 2010.
- Yang YL, Hsiao CC, Chen HY, et al.: Absence of biallelic TCRγ deletion predicts induction failure and poorer outcomes in childhood T-cell acute lymphoblastic leukemia. Pediatr Blood Cancer 58 (6): 846-51, 2012.
- Montefiori LE, Bendig S, Gu Z, et al.: Enhancer Hijacking Drives Oncogenic BCL11B Expression in Lineage-Ambiguous Stem Cell Leukemia. Cancer Discov 11 (11): 2846-2867, 2021.
- Di Giacomo D, La Starza R, Gorello P, et al.: 14q32 rearrangements deregulating BCL11B mark a distinct subgroup of T-lymphoid and myeloid immature acute leukemia. Blood 138 (9): 773-784, 2021.
- Béné MC: Biphenotypic, bilineal, ambiguous or mixed lineage: strange leukemias! Haematologica 94 (7): 891-3, 2009.
- Borowitz MJ, Béné MC, Harris NL, et al.: Acute leukaemias of ambiguous lineage. In: Swerdlow SH, Campo E, Harris NL, et al., eds.: WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th rev. ed. International Agency for Research on Cancer, 2017, pp 179-87.
- Davies SM, Bhatia S, Ross JA, et al.: Glutathione S-transferase genotypes, genetic susceptibility, and outcome of therapy in childhood acute lymphoblastic leukemia. Blood 100 (1): 67-71, 2002.
- Krajinovic M, Costea I, Chiasson S: Polymorphism of the thymidylate synthase gene and outcome of acute lymphoblastic leukaemia. Lancet 359 (9311): 1033-4, 2002.
- Krajinovic M, Lemieux-Blanchard E, Chiasson S, et al.: Role of polymorphisms in MTHFR and MTHFD1 genes in the outcome of childhood acute lymphoblastic leukemia. Pharmacogenomics J 4 (1): 66-72, 2004.
- Schmiegelow K, Forestier E, Kristinsson J, et al.: Thiopurine methyltransferase activity is related to the risk of relapse of childhood acute lymphoblastic leukemia: results from the NOPHO ALL-92 study. Leukemia 23 (3): 557-64, 2009.
- Relling MV, Hancock ML, Boyett JM, et al.: Prognostic importance of 6-mercaptopurine dose intensity in acute lymphoblastic leukemia. Blood 93 (9): 2817-23, 1999.
- Stanulla M, Schaeffeler E, Flohr T, et al.: Thiopurine methyltransferase (TPMT) genotype and early treatment response to mercaptopurine in childhood acute lymphoblastic leukemia. JAMA 293 (12): 1485-9, 2005.
- Yang JJ, Landier W, Yang W, et al.: Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. J Clin Oncol 33 (11): 1235-42, 2015.
- Relling MV, Hancock ML, Rivera GK, et al.: Mercaptopurine therapy intolerance and heterozygosity at the thiopurine S-methyltransferase gene locus. J Natl Cancer Inst 91 (23): 2001-8, 1999.
- Moriyama T, Nishii R, Perez-Andreu V, et al.: NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat Genet 48 (4): 367-73, 2016.
- Tanaka Y, Kato M, Hasegawa D, et al.: Susceptibility to 6-MP toxicity conferred by a NUDT15 variant in Japanese children with acute lymphoblastic leukaemia. Br J Haematol 171 (1): 109-15, 2015.
- Diouf B, Crews KR, Lew G, et al.: Association of an inherited genetic variant with vincristine-related peripheral neuropathy in children with acute lymphoblastic leukemia. JAMA 313 (8): 815-23, 2015.
- Yang JJ, Cheng C, Yang W, et al.: Genome-wide interrogation of germline genetic variation associated with treatment response in childhood acute lymphoblastic leukemia. JAMA 301 (4): 393-403, 2009.
- Gregers J, Christensen IJ, Dalhoff K, et al.: The association of reduced folate carrier 80G>A polymorphism to outcome in childhood acute lymphoblastic leukemia interacts with chromosome 21 copy number. Blood 115 (23): 4671-7, 2010.
- Radtke S, Zolk O, Renner B, et al.: Germline genetic variations in methotrexate candidate genes are associated with pharmacokinetics, toxicity, and outcome in childhood acute lymphoblastic leukemia. Blood 121 (26): 5145-53, 2013.
Risk-Based Treatment Assignment
Introduction to Risk-Based Treatment
Children with acute lymphoblastic leukemia (ALL) are usually treated according to risk groups defined by both clinical and laboratory features. The intensity of treatment required for cure varies substantially among subsets of children with ALL. Risk-based treatment assignment is used in children with ALL so that patients with favorable clinical and biological features who are likely to have a very good outcome with modest therapy can be spared more intensive and toxic treatment, while a more aggressive, potentially more toxic therapeutic approach is reserved for patients with a lower probability of long-term survival.[
Certain ALL study groups, such as the Children's Oncology Group (COG), use a more- or less-intensive induction regimen based on a subset of pretreatment factors, while other groups give a similar induction regimen to all patients.
Factors used by the COG to determine the intensity of induction include the following:
- Immunophenotype.
- The presence or absence of extramedullary disease.
- Steroid pretreatment.
- The presence or absence of Down syndrome.
- The National Cancer Institute (NCI) risk group classification.
The NCI risk group classification for B-ALL stratifies risk according to age and white blood cell (WBC) count, as follows:[
- Standard risk: WBC count less than 50,000/μL and age 1 to younger than 10 years.
- High risk: WBC count 50,000/μL or greater and/or age 10 years or older.
All study groups modify the intensity of postinduction therapy on the basis of a variety of prognostic factors, including NCI risk group, immunophenotype, early response determinations, and cytogenetics and genomic alterations.[
Risk-based treatment assignment requires the availability of prognostic factors that reliably predict outcome. For children with ALL, a number of factors have demonstrated prognostic value, some of which are described below.[
- Patient and clinical disease characteristics.
- Leukemic characteristics.
- Response to initial treatment.
As in any discussion of prognostic factors, the relative order of significance and the interrelationship of the variables are often treatment dependent and require multivariate analysis to determine which factors operate independently as prognostic variables. Because prognostic factors are treatment dependent, improvements in therapy may diminish or abrogate the significance of any of these prognostic factors.
A subset of the prognostic and clinical factors discussed below is used for the initial stratification of children with ALL for treatment assignment. For brief descriptions of the prognostic groupings currently applied in ongoing clinical trials in the United States, see the Prognostic (risk) groups under clinical evaluation section.
For information about important prognostic factors at relapse, see the Prognostic Factors After First Relapse of Childhood ALL section.
Prognostic Factors Affecting Risk-Based Treatment
Patient and clinical disease characteristics
Patient and clinical disease characteristics affecting prognosis include the following:
- Age at diagnosis.
- WBC count at diagnosis.
- Central nervous system (CNS) involvement at diagnosis.
- Testicular involvement at diagnosis.
- Down syndrome (trisomy 21).
- Sex.
- Race and ethnicity.
- Weight at diagnosis and during treatment.
Age at diagnosis
Age at diagnosis has strong prognostic significance in patients with B-ALL, reflecting the different underlying biology of ALL in different age groups.[
- Infants (younger than 1 year).
Infants with ALL have a particularly high risk of treatment failure. Treatment failure is most common in the following groups:
- Infants younger than 6 months (with an even poorer prognosis for those aged ≤90 days).[
9 ,10 ,11 ,12 ,13 ] - Infants with extremely high presenting leukocyte counts (>200,000–300,000 × 109 /L).[
10 ] - Infants with a poor response to a prednisone prophase.[
10 ] - Infants with a KMT2A gene rearrangement.[
9 ,10 ,11 ,12 ]
Up to 80% of infants with ALL have a translocation of 11q23 with numerous chromosome partners generating a KMT2A gene rearrangement.[
10 ,12 ,14 ,15 ] The most common rearrangement is KMT2A::AFF1 (t(4;11)(q21;q23)), but KMT2A rearrangements with many other translocation partners are observed. Infants with leukemia and KMT2A rearrangements typically have very high WBC counts and an increased incidence of CNS involvement. Event-free survival (EFS) and overall survival (OS) rates are poor. The 5-year EFS and OS rates are 35% to 40% for infants with KMT2A-rearranged ALL.[10 ,11 ,12 ]The frequency of KMT2A gene rearrangements is extremely high in infants younger than 6 months. From 6 months to 1 year, the incidence of KMT2A rearrangements decreases but remains significantly higher than that observed in older children.[
10 ,16 ] Blasts from infants with KMT2A rearrangements are often CD10 negative and express high levels of FLT3.[10 ,11 ,15 ,17 ] Conversely, infants whose leukemic cells show a germline KMT2A gene configuration frequently present with CD10-positive precursor-B immunophenotype. These infants have a significantly better outcome than do infants with ALL characterized by KMT2A rearrangements.[10 ,11 ,15 ,18 ]Black infants with ALL are significantly less likely to have KMT2A rearrangements than White infants.[
16 ]A comparison of the landscape of somatic variants in infants and older children with KMT2A-rearranged ALL revealed significant differences between the two groups. This result suggests distinctive age-related biological behaviors for KMT2A-rearranged ALL that may relate to the significantly poorer outcome for infants.[
19 ,20 ]For more information about infants with ALL, see the Infants With ALL section.
- Infants younger than 6 months (with an even poorer prognosis for those aged ≤90 days).[
- Young children (aged 1 to <10 years).
Young children (aged 1 to <10 years) with B-ALL have a better disease-free survival (DFS) rate than older children, adolescents, and infants.[
3 ,7 ,21 ,22 ,23 ] The improved prognosis in young children is at least partly explained by the more frequent occurrence of favorable cytogenetic features in the leukemic blasts, including hyperdiploidy with 51 to 65 chromosomes and/or favorable chromosome trisomies, or the ETV6::RUNX1 fusion (t(12;21)(p13;q22), previously known as the TEL::AML1 translocation).[7 ,24 ,25 ] - Adolescents and young adults (aged ≥10 years).
In general, the outcome of patients with B-ALL aged 10 years and older is inferior to that of patients aged 1 to younger than 10 years.[
26 ] Patients aged 10 to 15 years fare better than those who are aged 16 to 21 years at diagnosis who were treated with pediatric regimens.[8 ] However, the outcome for older adolescents has improved significantly over time.[27 ,28 ,29 ] Five-year survival rates for adolescents aged 15 to 19 years increased from 36% (1975–1984) to 78% (2011–2017).[30 ,31 ,32 ,33 ]Multiple retrospective studies have established that adolescents aged 16 to 21 years have a better outcome when treated on pediatric versus adult protocols.[
34 ,35 ,36 ] For more information about adolescents with ALL, see the Postinduction Treatment for Specific ALL Subgroups section.
WBC count at diagnosis
A WBC count of 50,000/µL is generally used as an operational cut point between better and poorer prognosis,[
The median WBC count at diagnosis is much higher for T-ALL (>50,000/µL) than for B-ALL (<10,000/µL), and there is no consistent effect of WBC count at diagnosis on prognosis for T-ALL.[
CNS involvement at diagnosis
The presence or absence of CNS leukemia at diagnosis has prognostic significance in both patients with B-ALL and T-ALL. Patients who have a nontraumatic diagnostic lumbar puncture may be placed into one of three categories according to the number of WBC/µL and the presence or absence of blasts on cytospin as follows:
- CNS1: Cerebrospinal fluid (CSF) that is cytospin negative for blasts regardless of WBC count.
- CNS2: CSF with fewer than 5 WBC/µL and cytospin positive for blasts.
- CNS3 (CNS disease): CSF with 5 or more WBC/µL and cytospin positive for blasts or clinical signs of CNS leukemia (i.e., facial nerve palsy, brain/eye involvement, or hypothalamic syndrome).
Children with B-ALL or T-ALL who present with CNS3 disease at diagnosis are at a higher risk of treatment failure (both within the CNS and systemically) than patients who are classified as CNS1 or CNS2.[
A traumatic lumbar puncture (≥10 erythrocytes/µL) that includes blasts at diagnosis has also been associated with increased risk of CNS relapse and overall poorer outcome in some studies,[
Most clinical trial groups have approached the treatment of CNS2 and traumatic lumbar puncture patients by using more intensive therapy, primarily additional doses of intrathecal therapy during induction.[
To determine whether a patient with a traumatic lumbar puncture (with blasts) should be treated as CNS3, the COG uses an algorithm relating the WBC and red blood cell counts in the spinal fluid and the peripheral blood.[
Testicular involvement at diagnosis
Overt testicular involvement at the time of diagnosis occurs in approximately 2% of males,[
In early ALL trials, testicular involvement at diagnosis was an adverse prognostic factor. With more aggressive initial therapy, however, it does not appear to have prognostic significance.[
The role of radiation therapy for testicular involvement is unclear. A study from St. Jude Children's Research Hospital (SJCRH) suggests that a good outcome can be achieved with aggressive conventional chemotherapy without radiation.[
Down syndrome (trisomy 21)
Outcomes in children with Down syndrome and ALL have often been somewhat inferior to outcomes in children without Down syndrome.[
- In a large retrospective study that included 653 patients with Down syndrome and ALL, patients with Down syndrome had a lower complete remission (CR) rate (97% vs. 99%, P < .001), higher cumulative incidence of relapse (26% vs. 15%, P < .001) and higher treatment-related mortality (7% vs. < 1%, P < .001) compared with patients without Down syndrome.[
70 ] Among the patients with Down syndrome, age younger than 6 years, WBC count of less than 10,000/µL, and the presence of the ETV6::RUNX1 fusion (observed in 8% of patients) were independent predictors of favorable EFS. - In a report from the COG, among patients with B-ALL who lacked KMT2A rearrangements, BCR::ABL1, ETV6::RUNX1, and hyperdiploidy with trisomies of chromosomes 4 and 10, the EFS and OS rates were similar in children with and without Down syndrome.[
69 ] - Certain genomic abnormalities, such as IKZF1 deletions, CRLF2 aberrations, and JAK variants, are seen more frequently in ALL arising in children with Down syndrome than in those without Down syndrome.[
72 ,73 ,74 ,75 ,76 ] Studies of children with Down syndrome and ALL suggest that the presence of IKZF1 deletions (but not CRLF2 aberrations or JAK variants) is associated with an inferior prognosis.[70 ,76 ,77 ] - A retrospective analysis included 130 patients with CRLF2-rearranged ALL and Down syndrome. Patients with the BCR::ABL1-like signature (25% of the CRLF2-rearranged cases) had an inferior outcome compared with those who lacked the BCR::ABL1-like signature (EFS rates, 39.5% ± 8.1% vs. 82.0% ± 4.4%; hazard ratio [HR], 5.27).[
71 ] - The IGH::IGF2BP1 gene fusion occurs in approximately 3% of patients with Down syndrome. This fusion is rare in patients with ALL who do not have Down syndrome. In one retrospective analysis, the fusion was associated with a relatively favorable outcome (EFS rate, 87.5% ± 1.7%).[
71 ]
Sex
In some studies, the prognosis for girls with ALL is slightly better than it is for boys with ALL.[
Race and ethnicity
Over the last several decades in the United States, survival rates in Black and Hispanic children with ALL have been somewhat lower than those in White children with ALL.[
The following factors associated with race and ethnicity influence survival:
- ALL subtype. The reason for better outcomes in White and Asian children than in Black and Hispanic children is at least partially explained by the different spectrum of ALL subtypes. For example, Black children have a higher relative incidence of T-ALL, lower rates of favorable genetic subtypes of B-ALL, and higher rates of the TFC3::PBX1 (t(1;19)) translocation. Hispanic and Latino children have a higher frequency of CRLF2 rearrangements and IKZF1 deletions.[
89 ,90 ,91 ] - Treatment adherence. Differences in outcome may also be related to treatment adherence, as illustrated by a study of adherence to oral mercaptopurine (6-MP) in maintenance therapy. In the first report from the study, there was an increased risk of relapse in Hispanic children compared with non-Hispanic White children, depending on the level of adherence, even when adjusting for other known variables. However, even with adherence rates of 90% or more, Hispanic children continued to demonstrate increased rates of relapse.[
92 ] In the second report from the study, adherence rates were shown to be significantly lower in Asian American and African American patients than in non-Hispanic White patients. A greater percentage of patients in these ethnic groups had adherence rates of less than 90%, which was associated with a 3.9-fold increased risk of relapse.[93 ] - Ancestry-related genomic variations. Ancestry-related genomic variations may also contribute to racial and ethnic disparities in both the incidence and outcome of ALL.[
94 ] For example, the differential presence of specific host polymorphisms in different racial and ethnic groups may contribute to outcome disparities, as illustrated by the occurrence of single nucleotide polymorphisms in the ARID5B gene that occur more frequently among Hispanic patients and are linked to both ALL susceptibility and to relapse hazard.[95 ] In a genome-wide association study (GWAS), the GATA3 variant, rs3824662, was associated with an increased risk of developing BCR::ABL1-like (Ph-like) ALL. Patients with this variant were at increased risk of high MRD at end-induction and at greater risk of relapse. The rs3824662 risk allele is associated with Native American genetic ancestry. The risk allele frequency was 52% in Guatemalan patients, 40% in U.S. Hispanic patients, and 14% in patients of European descent.[96 ]
Weight at diagnosis and during treatment
Studies of the impact of obesity on the outcome of ALL have had variable results. In most of these studies, obesity is defined as weight above the 95th percentile for age and height.
- Three studies did not demonstrate an independent effect of obesity on EFS.[
97 ][Level of evidence B4]; [98 ,99 ][Level of evidence C2] - Two studies showed obesity to be an independent prognostic factor only in patients older than 10 years or in patients with intermediate-risk or high-risk disease.[
100 ,101 ][Level of evidence C2] - The COG reported on the impact of obesity on outcome in 2,008 children, 14% of whom were obese, who were enrolled on a high-risk ALL trial (CCG-1961 [NCT00002812]).[
102 ][Level of evidence B4] Obesity was found to be an independent variable for inferior outcome compared with nonobese patients (5-year EFS rates, 64% vs. 74%; P = .002). However, obese patients at diagnosis who then lost weight during the premaintenance period of treatment had outcomes similar to patients with normal weight at diagnosis. - In a retrospective study of patients treated at a single institution, obesity at diagnosis was linked to an increased risk of having MRD at the end of induction and an inferior EFS.[
103 ][Level of evidence C2] - In a different retrospective study of 373 patients treated at a single institution, body mass index (BMI) at diagnosis was not associated with MRD at days 19 and 46, cumulative incidence of relapse, or EFS. OS was lower in patients with a high BMI, primarily resulting from treatment-related mortality and inferior salvage after relapse.[
104 ][Level of evidence C1] - In one study, obesity at diagnosis was associated with increased toxicity and truncated administration of asparaginase, especially in older children and adolescents.[
105 ] - In a study of 388 patients aged 15 to 50 years who were treated with Dana-Farber Cancer Institute (DFCI) ALL consortium regimens, greater BMI was associated with higher rates of relapse and nonrelapse mortality, as well as inferior OS. Higher BMI was associated with increased rates of hepatotoxicity and hyperglycemia. The deleterious effect of elevated BMI was more pronounced in older patients. Among patients aged 15 to 29 years at diagnosis (n = 254), the 4-year OS rate was 73% for those with high BMI, compared with 83% for those with BMI in the reference range (P = .09).[
106 ]
In a study of 762 pediatric patients with ALL (aged 2–17 years), the Dutch Childhood Oncology Group found that those who were underweight at diagnosis (defined as BMI standard deviation score < -1.8; 8% of the population) had an almost twofold higher risk of relapse compared with patients who were not underweight (after adjusting for risk group and age), although this did not result in a difference in EFS or OS. Patients with a decrease in BMI during the first 32 weeks of treatment had similar rates of relapse as other patients, but had significantly worse OS, primarily because of poorer salvage rates after relapse.[
Leukemic characteristics
Leukemic cell characteristics affecting prognosis include the following:
- Immunophenotype.
- Cytogenetics/genomic alterations.
Immunophenotype
The 2016 revision to the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia classifies ALL as either B-lymphoblastic leukemia or T-lymphoblastic leukemia, with further subdivisions based on molecular characteristics.[
Either B- or T-lymphoblastic leukemia can coexpress myeloid antigens. These cases need to be distinguished from leukemia of ambiguous lineage.
- B-ALL (WHO B-lymphoblastic leukemia).
Before 2008, the WHO classified B-lymphoblastic leukemia as precursor B-lymphoblastic leukemia, and this terminology is still frequently used in the literature of childhood ALL to distinguish it from mature B-cell ALL. Mature B-cell ALL is now termed Burkitt leukemia and requires different treatment than has been given for B-ALL (precursor B-cell ALL).
B-ALL, defined by the expression of CD19, HLA-DR, cytoplasmic CD79a, and other B-cell–associated antigens, accounts for 80% to 85% of childhood ALL. Approximately 90% of B-ALL cases express the CD10 surface antigen (formerly known as common ALL antigen). Absence of CD10 is often associated with KMT2A rearrangements, particularly t(4;11)(q21;q23), and a poor outcome.[
10 ,110 ] It is not clear whether CD10 negativity has any independent prognostic significance in the absence of a KMT2A gene rearrangement.[111 ]The major immunophenotypic subtypes of B-ALL are as follows:
- Common B-ALL (CD10 positive and no surface or cytoplasmic immunoglobulin [Ig]).
Approximately three-quarters of patients with B-ALL have the common precursor B-cell immunophenotype and have the best prognosis. Patients with favorable cytogenetics almost always show a common precursor B-cell immunophenotype.
- Pro-B ALL (CD10 negative and no surface or cytoplasmic Ig).
Approximately 5% of patients have the pro-B immunophenotype. Pro-B is the most common immunophenotype seen in infants and is often associated with KMT2A gene rearrangements.
- Pre-B ALL (presence of cytoplasmic Ig).
The leukemic cells of patients with pre-B ALL contain cytoplasmic Ig, and 25% of patients with pre-B ALL have the t(1;19)(q21;p13) translocation with the TCF3::PBX1 fusion.[
112 ,113 ]Approximately 3% of patients have transitional pre-B ALL with expression of surface Ig heavy chain in the absence of Ig light chain expression, MYC gene involvement, and L3 morphology. Patients with this phenotype respond well to therapy used for B-ALL.[
114 ] - Mature B-ALL (Burkitt lymphoma/leukemia).
Approximately 2% of patients present with mature B-cell leukemia (surface Ig expression, generally with French-American-British criteria L3 morphology and an 8q24 translocation involving MYC), also called Burkitt leukemia. The treatment for mature B-cell ALL is based on therapy for non-Hodgkin lymphoma and is completely different from the treatment for B-ALL. Rare cases of mature B-cell leukemia that lack surface Ig but have L3 morphology with MYC gene translocations should also be treated as mature B-cell leukemia.[
114 ] For more information about the treatment of children with mature B-cell lymphoma/leukemia and Burkitt lymphoma/leukemia, see Childhood Non-Hodgkin Lymphoma Treatment.A small number of cases of IG::MYC-translocated leukemias with precursor B-cell immunophenotype (e.g., absence of CD20 expression and surface Ig expression) have been reported.[
115 ] These cases presented in both children and adults. Like Burkitt lymphoma/leukemia, they had a male predominance and most patients showed L3 morphology. The cases lacked variants in genes recurrently altered in Burkitt lymphoma (e.g., ID3, CCND3, or MYC), whereas variants in RAS genes (frequently altered in B-ALL) were common. The clinical significance and optimal therapy of IG::MYC–translocated leukemias with precursor B-cell phenotype and molecular characteristics requires further study.
- Common B-ALL (CD10 positive and no surface or cytoplasmic immunoglobulin [Ig]).
- T-ALL.
T-ALL is defined by expression of the T-cell–associated antigens (cytoplasmic CD3, with CD7 plus CD2 or CD5) on leukemic blasts. T-ALL is frequently associated with a constellation of clinical features, including the following:[
21 ,39 ,81 ]- Male sex.
- Older age.
- Leukocytosis.
- Mediastinal mass.
While not true historically, with appropriately intensive therapy, children with T-ALL now have an outcome approaching that of children with B-ALL.[
21 ,39 ,42 ,43 ,81 ,116 ]There are few commonly accepted prognostic factors for patients with T-ALL. Conflicting data exist regarding the prognostic significance of presenting leukocyte counts in T-ALL.[
38 ,39 ,40 ,41 ,42 ,43 ,44 ,45 ,117 ] The presence or absence of a mediastinal mass at diagnosis has no prognostic significance. In patients with a mediastinal mass, the rate of regression of the mass lacks prognostic significance.[118 ]Early T-cell precursor (ETP) ALL.
ETP ALL, a distinct subset of childhood T-ALL, was initially defined by identifying T-ALL cases with gene expression profiles highly related to expression profiles for normal early T-cell precursors.[
119 ] The subset of T-ALL cases identified by these analyses represented 13% of all cases, and they were characterized by a distinctive immunophenotype (CD1a and CD8 negativity, with weak expression of CD5 and coexpression of stem cell or myeloid markers).Initial reports describing ETP ALL suggested that this subset of patients has a poorer prognosis than other patients with T-ALL.[
119 ,120 ,121 ] In addition, some studies have reported that these patients have a slower early response and higher frequency of induction failure.[45 ] Other studies have observed a more favorable outcome for patients with ETP ALL, including one study from the U.K. Medical Research Council that showed that the ETP ALL subgroup of patients had nonsignificantly inferior 5-year EFS rates compared with non-ETP patients (76% vs. 84%).[122 ] Similarly, in the COG AALL0434 [NCT00408005] trial, ETP status did not have a statistically significant impact on DFS (hazard ratio, 0.99; 95% CI, 0.59–1.67; P = .981) on multivariable analysis.[123 ,124 ] Further study in additional patient cohorts is needed to firmly establish the prognostic significance of early T-cell precursor ALL, but most ALL treatment groups do not change patient treatment on the basis of early T-cell precursor status. - Myeloid antigen expression.
Up to one-third of childhood ALL patients have leukemia cells that express myeloid-associated surface antigens. Myeloid-associated antigen expression appears to be associated with specific ALL subgroups, notably those with KMT2A rearrangements, ETV6::RUNX1, and BCR::ABL1.[
125 ,126 ,127 ] Patients with B-ALL who have gene rearrangements involving ZNF384 also commonly show myeloid antigen expression.[128 ,129 ] No independent adverse prognostic significance exists for myeloid-surface antigen expression.[125 ,126 ]For information about leukemia of ambiguous lineage, see the 2016 WHO Classification of Acute Leukemias of Ambiguous Lineage section.
Cytogenetics/genomic alterations
For information about B-ALL and T-ALL cytogenetics/genomics and gene polymorphisms in drug metabolic pathways, see the Cytogenetics/Genomics of Childhood ALL section.
Response to initial treatment
The rapidity with which leukemia cells are eliminated after initiation of treatment and the level of residual disease at the end of induction are associated with long-term outcome. Because treatment response is influenced by the drug sensitivity of leukemic cells and host pharmacodynamics and pharmacogenomics,[
- MRD determination in bone marrow at the end of induction and end of consolidation.
- Day 7 and day 14 bone marrow responses.
- Peripheral blood response to steroid prophase.
- Peripheral blood response to multiagent induction therapy.
- Peripheral blood MRD before end of induction (day 8, day 15).
- Persistent leukemia at the end of induction (induction failure).
MRD determination in bone marrow at the end of induction and end of consolidation
Morphological assessment of residual leukemia in blood or bone marrow is often difficult and is relatively insensitive. Traditionally, a cutoff of 5% blasts in the bone marrow (detected by light microscopy) has been used to determine remission status. This corresponds to a level of 1 in 20 malignant cells. To detect lower levels of leukemic cells in either blood or marrow, specialized techniques are required. Such techniques include polymerase chain reaction (PCR) assays, which determine unique Ig/T-cell receptor gene rearrangements and fusion transcripts produced by chromosome translocations, or flow cytometric assays, which detect leukemia-specific immunophenotypes. With these techniques, detection of as few as 1 leukemia cell in 100,000 normal cells is possible, and MRD at the level of 1 in 10,000 cells (1 × 10-4 or 0.01%) can be detected routinely.[
Multiple studies have demonstrated that end-induction MRD is an important, independent predictor of outcome in children and adolescents with B-lineage ALL.[
End-induction MRD is used by almost all groups as a factor in determining the intensity of postinduction treatment. Patients found to have higher MRD levels (typically >0.1% to 0.01%) are allocated to more intensive therapies.[
A study of 619 children with ALL compared the prognostic utility of MRD by flow cytometry with the more sensitive HTS assay. Using an end-induction MRD cut point level of 0.01%, HTS identified approximately 30% more cases as positive (i.e., >0.01%). Patients identified as positive by HTS but negative by flow cytometry had an intermediate prognosis, compared with patients categorized as either positive or negative by both methods. Patients meeting the criteria for standard-risk ALL with undetectable MRD by HTS had an especially good prognosis (5-year EFS rate, 98.1%).[
MRD levels obtained 10 to 12 weeks after the start of treatment (end-consolidation) have also been shown to be prognostically important. Patients with high levels of MRD at this time point have a significantly inferior EFS compared with other patients.[
- B-ALL. For patients with B-ALL, evaluating MRD at two time points (end-induction and end-consolidation) can identify the following three prognostically distinct patient subsets:[
136 ]- Low or undetectable end-induction MRD: Best prognosis.
- High end-induction MRD but low or negative end-consolidation MRD: Intermediate prognosis.
- High end-consolidation MRD (week 10–12 of therapy): Worst prognosis. The prognostic impact of end-consolidation MRD is modulated by NCI risk criteria. NCI high-risk patients with high end-consolidation MRD have DFS rates lower than NCI standard-risk patients who have similar MRD levels at this time point.[
140 ]
- T-ALL. There are fewer studies documenting the prognostic significance of MRD in patients with T-ALL. The United Kingdom Acute Lymphoblastic Leukaemia (UKALL) group reported that T-ALL patients with nondetectable end-induction MRD had excellent outcomes, while those with very high MRD levels (>5%) at the end of induction had a poor prognosis. However, for all other T-ALL patients, an association between end-induction MRD level and relapse risk was not found.[
137 ] The DFCI ALL consortium also reported that T-ALL patients with very low end-induction MRD (<10-4) had a very favorable outcome.[45 ]Another study also indicated that MRD at a later time point may be more prognostically significant in T-ALL.[
141 ] In the Associazione Italiana di Ematologia e Oncologia Pediatrica (AIEOP) ALL-BFM-2000 (NCT00430118) trial, MRD status at day 78 (week 12) was the most important predictor for relapse in patients with T-ALL.[141 ] Patients with detectable MRD at end-induction who had negative MRD by day 78 generally had a favorable prognosis, similar to that of patients who achieved MRD-negativity at the earlier end-induction time point.[141 ] The COG AALL1231 study confirmed the prognostic significance of end-consolidation marrow MRD for patients with T-ALL.[142 ]
MRD measurements, in conjunction with other presenting features, have also been used to identify subsets of patients with an extremely low risk of relapse. The COG reported a very favorable prognosis (5-year EFS rate, 97% ± 1%) for patients with precursor B-cell phenotype, NCI standard risk age/leukocyte count, CNS1 status, and favorable cytogenetic abnormalities (either high hyperdiploidy with favorable trisomies or the ETV6::RUNX1 fusion) who had less than 0.01% MRD levels at both day 8 (from peripheral blood) and end-induction (from bone marrow).[
Modifying therapy on the basis of MRD determination has been shown to improve outcome.
- The UKALL2003 (NCT00222612) study demonstrated that reduction of therapy (i.e., one rather than two courses of delayed intensification) did not adversely impact outcome in non-high–risk patients with favorable end-induction MRD.[
22 ][Level of evidence B1] In a randomized controlled trial, the UKALL2003 study also demonstrated improved EFS for standard-risk and intermediate-risk patients who received augmented therapy if end-induction MRD was greater than 0.01% (5-year EFS rates, 89.6% for augmented therapy vs. 82.8% for standard therapy).[144 ] - The Dutch ALL10 trial stratified patients into the following three risk groups on the basis of MRD after the first month of treatment and after the second cycle of chemotherapy:[
145 ][Level of evidence B4]- Standard risk (low MRD after the first month of treatment).
- Moderate risk (high MRD after the first month of treatment, low MRD after the second cycle of chemotherapy).
- High risk (high MRD after the second cycle of chemotherapy).
Compared with previous trials conducted by the same group, therapy was less intensive for standard-risk patients but more intensive for moderate-risk and high-risk patients. The overall 5-year EFS rate (87%) and OS rate (92%) were superior to the previous Dutch studies.
- In the DFCI ALL Consortium 05-001 trial, B-ALL patients with high end-induction MRD (defined as ≥1 × 10-3) were classified as very high risk regardless of other presenting characteristics. These patients received an intensified cytotoxic chemotherapy backbone. The 5-year DFS rate for these patients was 77%, significantly better than outcome for such patients on previous trials, when end-induction MRD was not used to stratify therapy.[
8 ]
Day 7 and day 14 bone marrow responses
Patients who have a rapid reduction in leukemia cells to less than 5% in their bone marrow within 7 or 14 days after the initiation of multiagent chemotherapy have a more favorable prognosis than patients who have slower clearance of leukemia cells from the bone marrow.[
Peripheral blood response to steroid prophase
Patients with a reduction in peripheral blast count to less than 1,000/µL after a 7-day induction prophase with prednisone and one dose of intrathecal methotrexate (a good prednisone response) have a more favorable prognosis than patients whose peripheral blast counts remain above 1,000/µL (a poor prednisone response).[
Peripheral blood response to multiagent induction therapy
Patients with persistent circulating leukemia cells at 7 to 10 days after the initiation of multiagent chemotherapy are at increased risk of relapse, compared with patients who have clearance of peripheral blasts within 1 week of therapy initiation.[
Peripheral blood MRD before end of induction (day 8, day 15)
MRD measured in peripheral blood obtained 1 week after the initiation of multiagent induction chemotherapy has also been evaluated as an early response-to-therapy prognostic factor.
- In a COG study involving nearly 2,000 children with ALL, the presence of MRD in the peripheral blood at day 8 was associated with adverse prognosis. Increasing MRD levels were associated with a progressively poorer outcome.[
134 ] - In multivariate analysis, end induction-therapy MRD was the most powerful prognostic factor, but day 8 peripheral blood MRD maintained its prognostic significance, as did NCI risk group and the presence of favorable trisomies. A smaller study assessed the prognostic significance of peripheral blood MRD at day 15 after 1 week of a steroid prophase and 1 week of multiagent induction therapy.[
150 ] This study also observed multivariate significance for peripheral blood MRD levels after 1 week of multiagent induction therapy.
Both studies identified a group of patients who achieved low MRD levels after 1 week of multiagent induction therapy who had a low rate of subsequent treatment failure.
Persistent leukemia at the end of induction (induction failure)
Nearly all children with ALL achieve complete morphological remission by the end of the first month of treatment. The presence of greater than 5% lymphoblasts by morphological assessment at the end of the induction phase is observed in 1% to 2% of children with ALL.[
Features associated with a higher risk of induction failure include the following:[
- T-cell phenotype.
- Higher WBC at diagnosis for patients with B-ALL.
- Older age.
- Unfavorable biology.
- KMT2A rearrangement.
- BCR::ABL1 rearrangement (before the use of tyrosine kinase inhibitors).
- Rearrangement of PDGFRB (most commonly EBF1::PDGFRB), commonly associated with the BCR::ABL1like subtype.[
153 ,156 ] These patients represent less than 1% of B-ALL cases in children but account for as much as 10% of induction failure cases.[153 ] In one retrospective study, 43 of 49 patients (88%) with PDGFRB fusions had end-induction MRD levels greater than 1%.[157 ]
In a large retrospective study, the OS rate of patients with induction failure was only 32%.[
Flow cytometry versus morphology
MRD is now being integrated with morphological assessment into the response to induction therapy, on the basis of studies that showed that patients with MRD levels above 5%, despite morphological CR, had outcomes similar to patients with morphological induction failure.
- In the UKALL2003 (NCT00222612) study, 59 of 3,113 patients (1.9%) had morphological induction failure.[
153 ]- The 5-year EFS rate was 51%, and the OS rate was 58%.
- 2.3% of patients had a morphological remission but had MRD of ≥5% measured by real-time quantitative IgH–T-cell receptor PCR. This group had a 5-year EFS rate of 47%, similar to those with morphological induction failure.
- The authors suggested that using both morphological and MRD criteria to define induction failure would more precisely identify patients with poor outcomes.
- A study of 9,350 patients enrolled on COG clinical trials between 2004 and 2014 compared characteristics of patients and their outcomes categorized by morphology (M1 vs. M2/M3) and MRD status assessed by flow cytometry (<5% vs. ≥5%). Morphological remission (M1 status) was achieved for 98.6% of B-ALL patients and 93.8% of T-ALL patients at the end of induction therapy.[
158 ]- Morphology and MRD were concordant in 97.4% of children. However, only 87.3% of T-ALL patients were M1 with MRD of <5%, while 97.8% of B-ALL patients were in concordant remission.
- Approximately 20% of patients (40 of 202) with M2/M3 morphology had MRD of <5%. B-ALL patients with M2/M3 morphology but MRD of <5% had a 5-year OS rate of 72.7%, which was inferior to that of patients concordantly in remission (5-year OS rate, 93.8%) but superior to that of patients with M3 marrow (5-year OS rate, 43.4%).
- Among B-ALL and T-ALL patients with M1 marrow, 0.9% of B-ALL patients and 6.9% of T-ALL patients had MRD of ≥5%. Their outcome was compared with that of patients with M1 marrow and MRD of <5% and are shown in Table 5 below.
- Table 5 shows that for children with B-ALL with M1 marrow and MRD of ≥5%, the 5-year EFS rate was significantly inferior to that of children concordantly in remission (59.1% vs. 87.1%) but was superior to that of children concordantly not in remission (M2 with MRD ≥5%: 5-year EFS rate, 39.1%).
- The impact on EFS for MRD of ≥5% for children with B-ALL in morphological remission was driven by NCI high-risk patients, as there was no significant difference in EFS between NCI standard-risk patients in morphological remission with or without MRD of ≥5%.
- Inferior EFS rates were also observed for patients with T-ALL with M1 marrow and MRD of ≥5% compared with those in concordant remission (87.6% vs. 80.3%). However, outcome for T-ALL patients not in remission (whether by morphology or MRD or both) was superior to that of comparable patients with B-ALL.
- Factors predictive of discordant MRD (≥5%) for patients in morphological remission at end of induction included age 10 years and older, WBC count at presentation of 50,000/µL or higher, neutral or unfavorable cytogenetics, and ETP ALL (for patients with T-ALL).
| Outcome | M1/MRD <5% | P valueb | M1/MRD ≥5% | P valuec | M2/MRD ≥5% | |
|---|---|---|---|---|---|---|
| HR = high risk; MRD = minimal residual disease; SR = standard risk. | ||||||
| a Adapted from Gupta et al.[ |
||||||
| b P value is comparing M1/MRD <5% with M1/MRD ≥5%. | ||||||
| c P value is comparing M1/MRD ≥5% with M2/MRD ≥5%. | ||||||
| Event-free survival rates: | ||||||
| B-ALL, overall | 87.1% ± 0.4% (n = 7,682) | <.0001 | 59.1% ± 6.5% (n = 66) | .009 | 39.1% ± 7.9% (n = 40) | |
| B-ALL, SR | 90.8% ± 0.4% (n = 5,000) | .25 | 85.9% ± 7.6% (n = 22) | .45 | 76.2% ± 15.2% (n = 9) | |
| B-ALL, HR | 80% ± 0.9% (n = 2,682) | <.0001 | 44.9% ± 8.3% (n = 44) | .05 | 29% ± 8.2% (n = 31) | |
| T-ALL | 87.6% ± 1.5% (n = 1,303) | .01 | 80.3% ± 7.3% (n = 97) | .13 | 62.7% ± 13.5% (n = 40) | |
| Overall survival rates: | ||||||
| B-ALL, overall | 93.8% ± 0.3% (n = 7,682) | <.0001 | 77.2% ± 5.6% (n = 66) | .01 | 59% ± 8.9% (n = 40) | |
| B-ALL, SR | 96.6% ± 0.3% (n = 5,000) | .24 | 95.5% ± 4.6% (n = 22 ) | .75 | 88.9% ± 12.1% (n = 9) | |
| B-ALL, HR | 88.4% ± 0.7% (n = 2,682) | <.0001 | 66.9% ± 8.3% (n = 44) | .06 | 51.4% ± 10.4% (n = 31) | |
| T-ALL | 91.9% ± 1.3% (n = 1,303) | .005 | 83.4% ± 6.8% (n = 97) | .34 | 76.7% ± 12.3% (n = 40) | |
Prognostic (Risk) Groups
For decades, clinical trial groups studying childhood ALL have used risk classification schemes to assign patients to therapeutic regimens on the basis of their estimated risk of treatment failure. Initial risk classification systems used clinical factors such as age and presenting WBC count. Response-to-therapy measures were subsequently added, with some groups using early morphological bone marrow response (e.g., at day 8 or day 15) and with other groups using response of circulating leukemia cells to single-agent prednisone. Contemporary risk classification systems continue to use clinical factors such as age and presenting WBC count and incorporate cytogenetics and genomic lesions of leukemia cells at diagnosis (e.g., favorable and unfavorable translocations) and response to therapy based on detection of MRD at end of induction (and in some cases at later time points).[
Children's Oncology Group (COG) risk groups
In COG protocols, children with ALL are initially stratified into treatment groups (with varying degrees of risk of treatment failure) on the basis of a subset of prognostic factors, including the following:
- Age.
- WBC count at diagnosis.
- Immunophenotype.
- Cytogenetics/genomic alterations.
- Presence of extramedullary disease.
- Down syndrome.
- Steroid pretreatment.
EFS rates exceed 85% in children meeting good-risk criteria (aged 1 to <10 years, WBC count <50,000/μL, and precursor B-cell immunophenotype). In children meeting high-risk criteria, EFS rates are approximately 75%.[
Patients who are at very high risk of treatment failure include the following:[
- Infants with KMT2A rearrangements.
- Patients with hypodiploidy (<44 chromosomes).
- Patients with initial induction failure.
Berlin-Frankfurt-Münster (BFM) risk groups
Since 2000, risk stratification on BFM protocols has been based on treatment response criteria, as well as biology. Treatment response is assessed primarily via MRD measurements at two time points, end-induction (time point 1, week 5) and end of the IB phase (similar to COG consolidation phase) at week 12 (time point 2). High MRD at both time points is defined as higher than 5 × 10-4.
The BFM defines 3 risk groups based on early response:[
- Standard risk: Patients who have negative MRD at both time points.
- Intermediate risk: Patients who have high MRD at time point 1 and negative MRD at time point 2.
- High risk: Patients with high MRD at time point 2. Patients with T-ALL with a poor response to the prednisone prophase are also considered high risk, regardless of subsequent MRD.
Biological factors used to stratify patients as high risk (regardless of MRD at either time point) include KMT2A::AFF1, TCF3::HLF, and hypodiploidy (<45 chromosomes). Patients with IKZF1-plus status (IKZF1 deletions that co-occurred with deletions in CDKN2A, CDKN2B, PAX5, or PAR1 in the absence of ERG deletion) [
Prognostic (risk) groups under clinical evaluation
- COG AALL1731 (NCT03914625) standard-risk and AALL1732 (NCT03959085) high-risk clinical trials: The COG classifies patients into six risk groups for patients with B-ALL (standard-risk favorable, standard-risk average, standard-risk high, high-risk favorable, high risk, and very high risk) on the basis of the following:
- Age and presenting leukocyte count (using NCI risk-group criteria).[
3 ]- NCI standard (low) risk: Includes children aged 1 year to <10 years with WBC <50,000/µL at the time of diagnosis.
- NCI high risk: Includes children aged ≥10 years and/or children who have WBC ≥50,000/µL at the time of diagnosis.
- Extramedullary disease (presence or absence of CNS and/or testicular leukemia).
- CNS1: Absence of blasts on CSF cytospin preparation, regardless of the number of WBCs.
- CNS2: Presence of <5 WBC/μL in CSF and cytospin positive for blasts; or traumatic LP, ≥5 WBC/μL, cytospin positive for blasts but negative by Steinherz/Bleyer algorithm.
- CNS3 is divided and defined as follows:
- CNS3a: <10 RBC/μL; ≥5 WBC/μL and cytospin positive for blasts.
- CNS3b: ≥10 RBC/μL; ≥5 WBC/μL and positive by Steinherz/Bleyer algorithm.
- CNS3c: Clinical signs of CNS leukemia (such as facial nerve palsy, brain/eye involvement or hypothalamic syndrome).
- Genomic alterations in leukemia cells.
- Day 8 peripheral blood MRD.
- Day 29 bone marrow morphological response and MRD.
- End of consolidation MRD.
- Steroid pretreatment.
Morphological assessment of early response in the bone marrow is no longer performed on days 8 and 15 of induction as part of risk stratification. Patients with T-cell phenotype are treated on separate trials and are not risk classified in this way.
For patients with B-ALL, the definitions of favorable, unfavorable, and neutral cytogenetics are as follows:
- Favorable cytogenetic features include the following:
- Hyperdiploidy with double trisomies of chromosomes 4 and 10 (double trisomy); or
- ETV6::RUNX1 fusion.
- Unfavorable cytogenetic features include the following:
- Hypodiploidy (<44 chromosomes or DNA index <0.81).
- KMT2A rearrangements.
- t(17;19)(q21-q22;p13.3) or resultant TCF3::HLF fusion transcript.
- Intrachromosomal amplification of chromosome 21 (iAMP21); and
- BCR::ABL1 (Ph+ or t(9;22)(q34;q11)). Patients with BCR::ABL1 ALL are treated on a separate clinical trial.
- Neutral cytogenetics: Lacking favorable and unfavorable cytogenetic features.
NCI standard-risk patients are divided into a highly favorable group (standard-risk favorable; 5-year DFS rate, >95%), a group with favorable outcome (standard-risk average; 5-year DFS rate, 90%–95%), and a group with a 5-year DFS rate below 90% (standard-risk high). Patients classified as standard-risk high receive postinduction backbone chemotherapy as per high-risk B-ALL regimens with intensified consolidation, interim maintenance, and reinduction therapy. Criteria for these three groups are provided in Table 6, Table 7, and Table 8 below.
Table 6. Standard-Risk Favorable B-ALL (Non-Down Syndrome and Down Syndrome) NCI Risk Group CNS Stage Steroid Pretreatmenta Favorable Genetics (ETV6::RUNX1or DT) PB MRD Day 8 BM MRD Day 29 BM = bone marrow; CNS = central nervous system; DT = double trisomy; MRD = minimal residual disease; NCI = National Cancer Institute; PB = peripheral blood; SR = standard risk. a Within one month prior to diagnosis. SR 1, 2 None Yes <1% <0.01% Table 7. Standard-Risk Average B-ALL (Non-Down Syndrome and Down Syndrome) NCI Risk Group CNS Stage ETV6::RUNX1 DT Neutral Cytogenetics PB MRD Day 8 BM MRD Day 29 BM = bone marrow; CNS = central nervous system; DT = double trisomy; MRD = minimal residual disease; NCI = National Cancer Institute; PB = peripheral blood; SR = standard risk. SR 1, 2 Yes to either No ≥1% <0.01% SR 1, 2 No Yes No Any ≥0.01 to <0.1% SR 1 No No Yes Any <0.01% Table 8. Standard-Risk High B-ALL NCI Risk Group CNS Stage ETV6::RUNX1 DT Neutral Cytogenetics Unfavorable Cytogenetics PB MRD Day 8 BM MRD Day 29 BM = bone marrow; CNS = central nervous system; DT = double trisomy; MRD = minimal residual disease; NCI = National Cancer Institute; PB = peripheral blood; SR = standard risk. SR 1, 2 Yes No No No Any ≥0.01% SR 1, 2 No Yes No No Any ≥0.1% SR 1 No No Yes No Any ≥0.01% SR 2 No No Yes No Any Any SR 1, 2 No No No Yes Any Any High-risk favorable B-ALL is defined by the characteristics in Table 9. These patients have an EFS rate higher than 90% on past COG clinical trials for high-risk patients.
Table 9. Characteristics of High-Risk Favorable B-ALL Patients NCI Risk Group Age (y) CNS Status Testicular Leukemia Steroid Pretreatment Favorable Genetics (ETV6::RUNX1or DT) Bone marrow MRD EOI HR <10 1 None ≤24 hoursa Yes <0.01% CNS = central nervous system; DT = double trisomy; EOI = end of induction; HR = high risk; MRD = minimal residual disease; NCI = National Cancer Institute. a Within two weeks of diagnosis. High-risk B-ALL is defined by the characteristics in Table 10. NCI standard-risk patients are elevated to high-risk status based on steroid pretreatment and CNS and/or testicular involvement.
Table 10. Characteristics of High-Risk B-ALL Patients NCI Risk Group Age (y) CNS and/or Testicular Leukemia Steroid Pretreatment Cytogenetics Bone marrow MRD EOI Bone marrow MRD EOC CNS = central nervous system; EOC = end of consolidation; EOI = end of induction; HR = high risk; MRD = minimal residual disease; N/A = not applicable; NCI = National Cancer Institute; SR = standard risk. a CNS3. b Philadelphia chromosome–positive (Ph+) ALL is excluded. c Only subjects with EOI bone marrow MRD ≥0.01% will have a bone marrow MRD assessment at EOC. d Within 2 weeks of diagnosis. e CNS2 or CNS3. SR <10 Yesa Any Anyb Any <1%c SR <10 No >24 hoursd Anyb Any <1%c HR ≥10 Any Any Anyb <0.01% N/A HR <10 Yese Any Anyb <0.01% N/A HR <10 No >24 hoursd Anyb <0.01% N/A HR <10 No ≤24 hoursd Neutral/unfavorableb <0.01% N/A HR Any Any Any Anyb ≥0.01% <0.01% NCI high-risk patients with end-of-consolidation marrow MRD ≥0.01% are classified as very high risk and are eligible for a chimeric antigen receptor (CAR) T-cell clinical trial in first remission (NCT03792633).
Patients with B-ALL and Down syndrome are classified into risk groups similar to other children, but Down syndrome patients classified as high risk receive a treatment regimen that is modified to reduce toxicity.
- Age and presenting leukocyte count (using NCI risk-group criteria).[
Current Clinical Trials
Use our
References:
- Hunger SP, Loh ML, Whitlock JA, et al.: Children's Oncology Group's 2013 blueprint for research: acute lymphoblastic leukemia. Pediatr Blood Cancer 60 (6): 957-63, 2013.
- Hunger SP, Mullighan CG: Acute Lymphoblastic Leukemia in Children. N Engl J Med 373 (16): 1541-52, 2015.
- Smith M, Arthur D, Camitta B, et al.: Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol 14 (1): 18-24, 1996.
- Schultz KR, Pullen DJ, Sather HN, et al.: Risk- and response-based classification of childhood B-precursor acute lymphoblastic leukemia: a combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children's Cancer Group (CCG). Blood 109 (3): 926-35, 2007.
- Jeha S, Coustan-Smith E, Pei D, et al.: Impact of tyrosine kinase inhibitors on minimal residual disease and outcome in childhood Philadelphia chromosome-positive acute lymphoblastic leukemia. Cancer 120 (10): 1514-9, 2014.
- Vrooman LM, Silverman LB: Childhood acute lymphoblastic leukemia: update on prognostic factors. Curr Opin Pediatr 21 (1): 1-8, 2009.
- Möricke A, Zimmermann M, Reiter A, et al.: Prognostic impact of age in children and adolescents with acute lymphoblastic leukemia: data from the trials ALL-BFM 86, 90, and 95. Klin Padiatr 217 (6): 310-20, 2005 Nov-Dec.
- Vrooman LM, Blonquist TM, Harris MH, et al.: Refining risk classification in childhood B acute lymphoblastic leukemia: results of DFCI ALL Consortium Protocol 05-001. Blood Adv 2 (12): 1449-1458, 2018.
- Reaman GH, Sposto R, Sensel MG, et al.: Treatment outcome and prognostic factors for infants with acute lymphoblastic leukemia treated on two consecutive trials of the Children's Cancer Group. J Clin Oncol 17 (2): 445-55, 1999.
- Pieters R, Schrappe M, De Lorenzo P, et al.: A treatment protocol for infants younger than 1 year with acute lymphoblastic leukaemia (Interfant-99): an observational study and a multicentre randomised trial. Lancet 370 (9583): 240-50, 2007.
- Hilden JM, Dinndorf PA, Meerbaum SO, et al.: Analysis of prognostic factors of acute lymphoblastic leukemia in infants: report on CCG 1953 from the Children's Oncology Group. Blood 108 (2): 441-51, 2006.
- Dreyer ZE, Hilden JM, Jones TL, et al.: Intensified chemotherapy without SCT in infant ALL: results from COG P9407 (Cohort 3). Pediatr Blood Cancer 62 (3): 419-26, 2015.
- Chessells JM, Harrison CJ, Watson SL, et al.: Treatment of infants with lymphoblastic leukaemia: results of the UK Infant Protocols 1987-1999. Br J Haematol 117 (2): 306-14, 2002.
- Isoyama K, Eguchi M, Hibi S, et al.: Risk-directed treatment of infant acute lymphoblastic leukaemia based on early assessment of MLL gene status: results of the Japan Infant Leukaemia Study (MLL96). Br J Haematol 118 (4): 999-1010, 2002.
- Nagayama J, Tomizawa D, Koh K, et al.: Infants with acute lymphoblastic leukemia and a germline MLL gene are highly curable with use of chemotherapy alone: results from the Japan Infant Leukemia Study Group. Blood 107 (12): 4663-5, 2006.
- Sam TN, Kersey JH, Linabery AM, et al.: MLL gene rearrangements in infant leukemia vary with age at diagnosis and selected demographic factors: a Children's Oncology Group (COG) study. Pediatr Blood Cancer 58 (6): 836-9, 2012.
- Stam RW, Schneider P, de Lorenzo P, et al.: Prognostic significance of high-level FLT3 expression in MLL-rearranged infant acute lymphoblastic leukemia. Blood 110 (7): 2774-5, 2007.
- De Lorenzo P, Moorman AV, Pieters R, et al.: Cytogenetics and outcome of infants with acute lymphoblastic leukemia and absence of MLL rearrangements. Leukemia 28 (2): 428-30, 2014.
- Kang H, Wilson CS, Harvey RC, et al.: Gene expression profiles predictive of outcome and age in infant acute lymphoblastic leukemia: a Children's Oncology Group study. Blood 119 (8): 1872-81, 2012.
- Andersson AK, Ma J, Wang J, et al.: The landscape of somatic mutations in infant MLL-rearranged acute lymphoblastic leukemias. Nat Genet 47 (4): 330-7, 2015.
- Schrappe M, Reiter A, Ludwig WD, et al.: Improved outcome in childhood acute lymphoblastic leukemia despite reduced use of anthracyclines and cranial radiotherapy: results of trial ALL-BFM 90. German-Austrian-Swiss ALL-BFM Study Group. Blood 95 (11): 3310-22, 2000.
- Vora A, Goulden N, Wade R, et al.: Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): a randomised controlled trial. Lancet Oncol 14 (3): 199-209, 2013.
- Place AE, Stevenson KE, Vrooman LM, et al.: Intravenous pegylated asparaginase versus intramuscular native Escherichia coli L-asparaginase in newly diagnosed childhood acute lymphoblastic leukaemia (DFCI 05-001): a randomised, open-label phase 3 trial. Lancet Oncol 16 (16): 1677-90, 2015.
- Forestier E, Schmiegelow K; on behalf of the Nordic Society of Paediatric Haematology and Oncology NOPHO: The incidence peaks of the childhood acute leukemias reflect specific cytogenetic aberrations. J Pediatr Hematol Oncol 28 (8): 486-95, 2006.
- Dastugue N, Suciu S, Plat G, et al.: Hyperdiploidy with 58-66 chromosomes in childhood B-acute lymphoblastic leukemia is highly curable: 58951 CLG-EORTC results. Blood 121 (13): 2415-23, 2013.
- Burke MJ, Devidas M, Chen Z, et al.: Outcomes in adolescent and young adult patients (16 to 30 years) compared to younger patients treated for high-risk B-lymphoblastic leukemia: report from Children's Oncology Group Study AALL0232. Leukemia 36 (3): 648-655, 2022.
- Nachman JB, La MK, Hunger SP, et al.: Young adults with acute lymphoblastic leukemia have an excellent outcome with chemotherapy alone and benefit from intensive postinduction treatment: a report from the children's oncology group. J Clin Oncol 27 (31): 5189-94, 2009.
- Pulte D, Gondos A, Brenner H: Improvement in survival in younger patients with acute lymphoblastic leukemia from the 1980s to the early 21st century. Blood 113 (7): 1408-11, 2009.
- Pui CH, Pei D, Campana D, et al.: Improved prognosis for older adolescents with acute lymphoblastic leukemia. J Clin Oncol 29 (4): 386-91, 2011.
- Childhood cancer. In: Howlader N, Noone AM, Krapcho M, et al., eds.: SEER Cancer Statistics Review, 1975-2010. National Cancer Institute, 2013, Section 28.
Also available online . Last accessed August 21, 2023. - Childhood cancer by the ICCC. In: Howlader N, Noone AM, Krapcho M, et al., eds.: SEER Cancer Statistics Review, 1975-2010. National Cancer Institute, 2013, Section 29.
Also available online . Last accessed August 21, 2023. - Smith MA, Ries LA, Gurney JG, et al.: Leukemia. In: Ries LA, Smith MA, Gurney JG, et al., eds.: Cancer incidence and survival among children and adolescents: United States SEER Program 1975-1995. National Cancer Institute, SEER Program, 1999. NIH Pub.No. 99-4649, pp 17-34.
Also available online . Last accessed August 11, 2022. - National Cancer Institute: NCCR*Explorer: An interactive website for NCCR cancer statistics. Bethesda, MD: National Cancer Institute.
Available online . Last accessed August 23, 2024. - de Bont JM, Holt B, Dekker AW, et al.: Significant difference in outcome for adolescents with acute lymphoblastic leukemia treated on pediatric vs adult protocols in the Netherlands. Leukemia 18 (12): 2032-5, 2004.
- Boissel N, Auclerc MF, Lhéritier V, et al.: Should adolescents with acute lymphoblastic leukemia be treated as old children or young adults? Comparison of the French FRALLE-93 and LALA-94 trials. J Clin Oncol 21 (5): 774-80, 2003.
- Stock W, La M, Sanford B, et al.: What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? A comparison of Children's Cancer Group and Cancer and Leukemia Group B studies. Blood 112 (5): 1646-54, 2008.
- Hastings C, Gaynon PS, Nachman JB, et al.: Increased post-induction intensification improves outcome in children and adolescents with a markedly elevated white blood cell count (≥200 × 10(9) /l) with T cell acute lymphoblastic leukaemia but not B cell disease: a report from the Children's Oncology Group. Br J Haematol 168 (4): 533-46, 2015.
- Pullen J, Shuster JJ, Link M, et al.: Significance of commonly used prognostic factors differs for children with T cell acute lymphocytic leukemia (ALL), as compared to those with B-precursor ALL. A Pediatric Oncology Group (POG) study. Leukemia 13 (11): 1696-707, 1999.
- Goldberg JM, Silverman LB, Levy DE, et al.: Childhood T-cell acute lymphoblastic leukemia: the Dana-Farber Cancer Institute acute lymphoblastic leukemia consortium experience. J Clin Oncol 21 (19): 3616-22, 2003.
- Silverman LB, Stevenson KE, O'Brien JE, et al.: Long-term results of Dana-Farber Cancer Institute ALL Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1985-2000). Leukemia 24 (2): 320-34, 2010.
- Pui CH, Pei D, Sandlund JT, et al.: Long-term results of St Jude Total Therapy Studies 11, 12, 13A, 13B, and 14 for childhood acute lymphoblastic leukemia. Leukemia 24 (2): 371-82, 2010.
- Gaynon PS, Angiolillo AL, Carroll WL, et al.: Long-term results of the children's cancer group studies for childhood acute lymphoblastic leukemia 1983-2002: a Children's Oncology Group Report. Leukemia 24 (2): 285-97, 2010.
- Möricke A, Zimmermann M, Reiter A, et al.: Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia 24 (2): 265-84, 2010.
- Vaitkevičienė G, Forestier E, Hellebostad M, et al.: High white blood cell count at diagnosis of childhood acute lymphoblastic leukaemia: biological background and prognostic impact. Results from the NOPHO ALL-92 and ALL-2000 studies. Eur J Haematol 86 (1): 38-46, 2011.
- Burns MA, Place AE, Stevenson KE, et al.: Identification of prognostic factors in childhood T-cell acute lymphoblastic leukemia: Results from DFCI ALL Consortium Protocols 05-001 and 11-001. Pediatr Blood Cancer 68 (1): e28719, 2021.
- Bürger B, Zimmermann M, Mann G, et al.: Diagnostic cerebrospinal fluid examination in children with acute lymphoblastic leukemia: significance of low leukocyte counts with blasts or traumatic lumbar puncture. J Clin Oncol 21 (2): 184-8, 2003.
- Vora A, Andreano A, Pui CH, et al.: Influence of Cranial Radiotherapy on Outcome in Children With Acute Lymphoblastic Leukemia Treated With Contemporary Therapy. J Clin Oncol 34 (9): 919-26, 2016.
- Gossai NP, Devidas M, Chen Z, et al.: Central nervous system status is prognostic in T-cell acute lymphoblastic leukemia: a Children's Oncology Group report. Blood 141 (15): 1802-1811, 2023.
- Mahmoud HH, Rivera GK, Hancock ML, et al.: Low leukocyte counts with blast cells in cerebrospinal fluid of children with newly diagnosed acute lymphoblastic leukemia. N Engl J Med 329 (5): 314-9, 1993.
- Winick N, Devidas M, Chen S, et al.: Impact of Initial CSF Findings on Outcome Among Patients With National Cancer Institute Standard- and High-Risk B-Cell Acute Lymphoblastic Leukemia: A Report From the Children's Oncology Group. J Clin Oncol 35 (22): 2527-2534, 2017.
- Sirvent N, Suciu S, Rialland X, et al.: Prognostic significance of the initial cerebro-spinal fluid (CSF) involvement of children with acute lymphoblastic leukaemia (ALL) treated without cranial irradiation: results of European Organization for Research and Treatment of Cancer (EORTC) Children Leukemia Group study 58881. Eur J Cancer 47 (2): 239-47, 2011.
- te Loo DM, Kamps WA, van der Does-van den Berg A, et al.: Prognostic significance of blasts in the cerebrospinal fluid without pleiocytosis or a traumatic lumbar puncture in children with acute lymphoblastic leukemia: experience of the Dutch Childhood Oncology Group. J Clin Oncol 24 (15): 2332-6, 2006.
- Gilchrist GS, Tubergen DG, Sather HN, et al.: Low numbers of CSF blasts at diagnosis do not predict for the development of CNS leukemia in children with intermediate-risk acute lymphoblastic leukemia: a Childrens Cancer Group report. J Clin Oncol 12 (12): 2594-600, 1994.
- Gajjar A, Harrison PL, Sandlund JT, et al.: Traumatic lumbar puncture at diagnosis adversely affects outcome in childhood acute lymphoblastic leukemia. Blood 96 (10): 3381-4, 2000.
- Matloub Y, Bostrom BC, Hunger SP, et al.: Escalating intravenous methotrexate improves event-free survival in children with standard-risk acute lymphoblastic leukemia: a report from the Children's Oncology Group. Blood 118 (2): 243-51, 2011.
- Pui CH, Campana D, Pei D, et al.: Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med 360 (26): 2730-41, 2009.
- Levinsen M, Taskinen M, Abrahamsson J, et al.: Clinical features and early treatment response of central nervous system involvement in childhood acute lymphoblastic leukemia. Pediatr Blood Cancer 61 (8): 1416-21, 2014.
- Jeha S, Pei D, Choi J, et al.: Improved CNS Control of Childhood Acute Lymphoblastic Leukemia Without Cranial Irradiation: St Jude Total Therapy Study 16. J Clin Oncol 37 (35): 3377-3391, 2019.
- Cherlow JM, Sather H, Steinherz P, et al.: Craniospinal irradiation for acute lymphoblastic leukemia with central nervous system disease at diagnosis: a report from the Children's Cancer Group. Int J Radiat Oncol Biol Phys 36 (1): 19-27, 1996.
- Hijiya N, Liu W, Sandlund JT, et al.: Overt testicular disease at diagnosis of childhood acute lymphoblastic leukemia: lack of therapeutic role of local irradiation. Leukemia 19 (8): 1399-403, 2005.
- Sirvent N, Suciu S, Bertrand Y, et al.: Overt testicular disease (OTD) at diagnosis is not associated with a poor prognosis in childhood acute lymphoblastic leukemia: results of the EORTC CLG Study 58881. Pediatr Blood Cancer 49 (3): 344-8, 2007.
- Bassal M, La MK, Whitlock JA, et al.: Lymphoblast biology and outcome among children with Down syndrome and ALL treated on CCG-1952. Pediatr Blood Cancer 44 (1): 21-8, 2005.
- Zeller B, Gustafsson G, Forestier E, et al.: Acute leukaemia in children with Down syndrome: a population-based Nordic study. Br J Haematol 128 (6): 797-804, 2005.
- Whitlock JA, Sather HN, Gaynon P, et al.: Clinical characteristics and outcome of children with Down syndrome and acute lymphoblastic leukemia: a Children's Cancer Group study. Blood 106 (13): 4043-9, 2005.
- Arico M, Ziino O, Valsecchi MG, et al.: Acute lymphoblastic leukemia and Down syndrome: presenting features and treatment outcome in the experience of the Italian Association of Pediatric Hematology and Oncology (AIEOP). Cancer 113 (3): 515-21, 2008.
- Lundin C, Forestier E, Klarskov Andersen M, et al.: Clinical and genetic features of pediatric acute lymphoblastic leukemia in Down syndrome in the Nordic countries. J Hematol Oncol 7 (1): 32, 2014.
- Athale UH, Puligandla M, Stevenson KE, et al.: Outcome of children and adolescents with Down syndrome treated on Dana-Farber Cancer Institute Acute Lymphoblastic Leukemia Consortium protocols 00-001 and 05-001. Pediatr Blood Cancer 65 (10): e27256, 2018.
- Matloub Y, Rabin KR, Ji L, et al.: Excellent long-term survival of children with Down syndrome and standard-risk ALL: a report from the Children's Oncology Group. Blood Adv 3 (11): 1647-1656, 2019.
- Maloney KW, Carroll WL, Carroll AJ, et al.: Down syndrome childhood acute lymphoblastic leukemia has a unique spectrum of sentinel cytogenetic lesions that influences treatment outcome: a report from the Children's Oncology Group. Blood 116 (7): 1045-50, 2010.
- Buitenkamp TD, Izraeli S, Zimmermann M, et al.: Acute lymphoblastic leukemia in children with Down syndrome: a retrospective analysis from the Ponte di Legno study group. Blood 123 (1): 70-7, 2014.
- Li Z, Chang TC, Junco JJ, et al.: Genomic landscape of Down syndrome-associated acute lymphoblastic leukemia. Blood 142 (2): 172-184, 2023.
- Mullighan CG, Collins-Underwood JR, Phillips LA, et al.: Rearrangement of CRLF2 in B-progenitor- and Down syndrome-associated acute lymphoblastic leukemia. Nat Genet 41 (11): 1243-6, 2009.
- Bercovich D, Ganmore I, Scott LM, et al.: Mutations of JAK2 in acute lymphoblastic leukaemias associated with Down's syndrome. Lancet 372 (9648): 1484-92, 2008.
- Gaikwad A, Rye CL, Devidas M, et al.: Prevalence and clinical correlates of JAK2 mutations in Down syndrome acute lymphoblastic leukaemia. Br J Haematol 144 (6): 930-2, 2009.
- Kearney L, Gonzalez De Castro D, Yeung J, et al.: Specific JAK2 mutation (JAK2R683) and multiple gene deletions in Down syndrome acute lymphoblastic leukemia. Blood 113 (3): 646-8, 2009.
- Buitenkamp TD, Pieters R, Gallimore NE, et al.: Outcome in children with Down's syndrome and acute lymphoblastic leukemia: role of IKZF1 deletions and CRLF2 aberrations. Leukemia 26 (10): 2204-11, 2012.
- Hanada I, Terui K, Ikeda F, et al.: Gene alterations involving the CRLF2-JAK pathway and recurrent gene deletions in Down syndrome-associated acute lymphoblastic leukemia in Japan. Genes Chromosomes Cancer 53 (11): 902-10, 2014.
- Pui CH, Boyett JM, Relling MV, et al.: Sex differences in prognosis for children with acute lymphoblastic leukemia. J Clin Oncol 17 (3): 818-24, 1999.
- Shuster JJ, Wacker P, Pullen J, et al.: Prognostic significance of sex in childhood B-precursor acute lymphoblastic leukemia: a Pediatric Oncology Group Study. J Clin Oncol 16 (8): 2854-63, 1998.
- Chessells JM, Richards SM, Bailey CC, et al.: Gender and treatment outcome in childhood lymphoblastic leukaemia: report from the MRC UKALL trials. Br J Haematol 89 (2): 364-72, 1995.
- Silverman LB, Gelber RD, Dalton VK, et al.: Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood 97 (5): 1211-8, 2001.
- Hunger SP, Lu X, Devidas M, et al.: Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children's oncology group. J Clin Oncol 30 (14): 1663-9, 2012.
- Gupta S, Teachey DT, Chen Z, et al.: Sex-based disparities in outcome in pediatric acute lymphoblastic leukemia: a Children's Oncology Group report. Cancer 128 (9): 1863-1870, 2022.
- Bhatia S: Influence of race and socioeconomic status on outcome of children treated for childhood acute lymphoblastic leukemia. Curr Opin Pediatr 16 (1): 9-14, 2004.
- Kadan-Lottick NS, Ness KK, Bhatia S, et al.: Survival variability by race and ethnicity in childhood acute lymphoblastic leukemia. JAMA 290 (15): 2008-14, 2003.
- Tai EW, Ward KC, Bonaventure A, et al.: Survival among children diagnosed with acute lymphoblastic leukemia in the United States, by race and age, 2001 to 2009: Findings from the CONCORD-2 study. Cancer 123 (Suppl 24): 5178-5189, 2017.
- Kahn JM, Cole PD, Blonquist TM, et al.: An investigation of toxicities and survival in Hispanic children and adolescents with ALL: Results from the Dana-Farber Cancer Institute ALL Consortium protocol 05-001. Pediatr Blood Cancer 65 (3): , 2018.
- Gupta S, Dai Y, Chen Z, et al.: Racial and ethnic disparities in childhood and young adult acute lymphocytic leukaemia: secondary analyses of eight Children's Oncology Group cohort trials. Lancet Haematol 10 (2): e129-e141, 2023.
- Lee SHR, Antillon-Klussmann F, Pei D, et al.: Association of Genetic Ancestry With the Molecular Subtypes and Prognosis of Childhood Acute Lymphoblastic Leukemia. JAMA Oncol 8 (3): 354-363, 2022.
- Harvey RC, Mullighan CG, Chen IM, et al.: Rearrangement of CRLF2 is associated with mutation of JAK kinases, alteration of IKZF1, Hispanic/Latino ethnicity, and a poor outcome in pediatric B-progenitor acute lymphoblastic leukemia. Blood 115 (26): 5312-21, 2010.
- Raca G, Abdel-Azim H, Yue F, et al.: Increased Incidence of IKZF1 deletions and IGH-CRLF2 translocations in B-ALL of Hispanic/Latino children-a novel health disparity. Leukemia 35 (8): 2399-2402, 2021.
- Bhatia S, Landier W, Shangguan M, et al.: Nonadherence to oral mercaptopurine and risk of relapse in Hispanic and non-Hispanic white children with acute lymphoblastic leukemia: a report from the children's oncology group. J Clin Oncol 30 (17): 2094-101, 2012.
- Bhatia S, Landier W, Hageman L, et al.: 6MP adherence in a multiracial cohort of children with acute lymphoblastic leukemia: a Children's Oncology Group study. Blood 124 (15): 2345-53, 2014.
- Yang JJ, Cheng C, Devidas M, et al.: Ancestry and pharmacogenomics of relapse in acute lymphoblastic leukemia. Nat Genet 43 (3): 237-41, 2011.
- Xu H, Cheng C, Devidas M, et al.: ARID5B genetic polymorphisms contribute to racial disparities in the incidence and treatment outcome of childhood acute lymphoblastic leukemia. J Clin Oncol 30 (7): 751-7, 2012.
- Perez-Andreu V, Roberts KG, Harvey RC, et al.: Inherited GATA3 variants are associated with Ph-like childhood acute lymphoblastic leukemia and risk of relapse. Nat Genet 45 (12): 1494-8, 2013.
- Aldhafiri FK, McColl JH, Reilly JJ: Prognostic significance of being overweight and obese at diagnosis in children with acute lymphoblastic leukemia. J Pediatr Hematol Oncol 36 (3): 234-6, 2014.
- Baillargeon J, Langevin AM, Lewis M, et al.: Obesity and survival in a cohort of predominantly Hispanic children with acute lymphoblastic leukemia. J Pediatr Hematol Oncol 28 (9): 575-8, 2006.
- Hijiya N, Panetta JC, Zhou Y, et al.: Body mass index does not influence pharmacokinetics or outcome of treatment in children with acute lymphoblastic leukemia. Blood 108 (13): 3997-4002, 2006.
- Butturini AM, Dorey FJ, Lange BJ, et al.: Obesity and outcome in pediatric acute lymphoblastic leukemia. J Clin Oncol 25 (15): 2063-9, 2007.
- Gelelete CB, Pereira SH, Azevedo AM, et al.: Overweight as a prognostic factor in children with acute lymphoblastic leukemia. Obesity (Silver Spring) 19 (9): 1908-11, 2011.
- Orgel E, Sposto R, Malvar J, et al.: Impact on survival and toxicity by duration of weight extremes during treatment for pediatric acute lymphoblastic leukemia: A report from the Children's Oncology Group. J Clin Oncol 32 (13): 1331-7, 2014.
- Orgel E, Tucci J, Alhushki W, et al.: Obesity is associated with residual leukemia following induction therapy for childhood B-precursor acute lymphoblastic leukemia. Blood 124 (26): 3932-8, 2014.
- Eissa HM, Zhou Y, Panetta JC, et al.: The effect of body mass index at diagnosis on clinical outcome in children with newly diagnosed acute lymphoblastic leukemia. Blood Cancer J 7 (2): e531, 2017.
- Egnell C, Heyman M, Jónsson ÓG, et al.: Obesity as a predictor of treatment-related toxicity in children with acute lymphoblastic leukaemia. Br J Haematol 196 (5): 1239-1247, 2022.
- Shimony S, Flamand Y, Valtis YK, et al.: Effect of BMI on toxicities and survival among adolescents and young adults treated on DFCI Consortium ALL trials. Blood Adv 7 (18): 5234-5245, 2023.
- den Hoed MA, Pluijm SM, de Groot-Kruseman HA, et al.: The negative impact of being underweight and weight loss on survival of children with acute lymphoblastic leukemia. Haematologica 100 (1): 62-9, 2015.
- Swerdlow SH, Campo E, Harris NL, et al., eds.: WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th rev. ed. International Agency for Research on Cancer, 2017.
- Arber DA, Orazi A, Hasserjian R, et al.: The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127 (20): 2391-405, 2016.
- Pui CH, Chessells JM, Camitta B, et al.: Clinical heterogeneity in childhood acute lymphoblastic leukemia with 11q23 rearrangements. Leukemia 17 (4): 700-6, 2003.
- Möricke A, Ratei R, Ludwig WD, et al.: Prognostic factors in CD10 negative precursor b-cell acute lymphoblastic leukemia in children: data from three consecutive trials ALL-BFM 86, 90, and 95. [Abstract] Blood 104 (11): A-1957, 540a, 2004.
- Hunger SP: Chromosomal translocations involving the E2A gene in acute lymphoblastic leukemia: clinical features and molecular pathogenesis. Blood 87 (4): 1211-24, 1996.
- Uckun FM, Sensel MG, Sather HN, et al.: Clinical significance of translocation t(1;19) in childhood acute lymphoblastic leukemia in the context of contemporary therapies: a report from the Children's Cancer Group. J Clin Oncol 16 (2): 527-35, 1998.
- Koehler M, Behm FG, Shuster J, et al.: Transitional pre-B-cell acute lymphoblastic leukemia of childhood is associated with favorable prognostic clinical features and an excellent outcome: a Pediatric Oncology Group study. Leukemia 7 (12): 2064-8, 1993.
- Wagener R, López C, Kleinheinz K, et al.: IG-MYC+ neoplasms with precursor B-cell phenotype are molecularly distinct from Burkitt lymphomas. Blood 132 (21): 2280-2285, 2018.
- Winter SS, Dunsmore KP, Devidas M, et al.: Improved Survival for Children and Young Adults With T-Lineage Acute Lymphoblastic Leukemia: Results From the Children's Oncology Group AALL0434 Methotrexate Randomization. J Clin Oncol 36 (29): 2926-2934, 2018.
- Slack JL, Arthur DC, Lawrence D, et al.: Secondary cytogenetic changes in acute promyelocytic leukemia--prognostic importance in patients treated with chemotherapy alone and association with the intron 3 breakpoint of the PML gene: a Cancer and Leukemia Group B study. J Clin Oncol 15 (5): 1786-95, 1997.
- Attarbaschi A, Mann G, Dworzak M, et al.: Mediastinal mass in childhood T-cell acute lymphoblastic leukemia: significance and therapy response. Med Pediatr Oncol 39 (6): 558-65, 2002.
- Coustan-Smith E, Mullighan CG, Onciu M, et al.: Early T-cell precursor leukaemia: a subtype of very high-risk acute lymphoblastic leukaemia. Lancet Oncol 10 (2): 147-56, 2009.
- Ma M, Wang X, Tang J, et al.: Early T-cell precursor leukemia: a subtype of high risk childhood acute lymphoblastic leukemia. Front Med 6 (4): 416-20, 2012.
- Inukai T, Kiyokawa N, Campana D, et al.: Clinical significance of early T-cell precursor acute lymphoblastic leukaemia: results of the Tokyo Children's Cancer Study Group Study L99-15. Br J Haematol 156 (3): 358-65, 2012.
- Patrick K, Wade R, Goulden N, et al.: Outcome for children and young people with Early T-cell precursor acute lymphoblastic leukaemia treated on a contemporary protocol, UKALL 2003. Br J Haematol 166 (3): 421-4, 2014.
- Dunsmore KP, Winter SS, Devidas M, et al.: Children's Oncology Group AALL0434: A Phase III Randomized Clinical Trial Testing Nelarabine in Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia. J Clin Oncol 38 (28): 3282-3293, 2020.
- Wood BL, Winter SS, Dunsmore KP, et al.: T-lymphoblastic leukemia (T-ALL) shows excellent outcome, lack of significance of the early thymic precursor (ETP) immunophenotype, and validation of the prognostic value of end-induction minimal residual disease (MRD) in Children's Oncology Group (COG) study AALL0434. [Abstract] Blood 124 (21): A-1, 2014.
Also available online . Last accessed June 04, 2021. - Pui CH, Rubnitz JE, Hancock ML, et al.: Reappraisal of the clinical and biologic significance of myeloid-associated antigen expression in childhood acute lymphoblastic leukemia. J Clin Oncol 16 (12): 3768-73, 1998.
- Uckun FM, Sather HN, Gaynon PS, et al.: Clinical features and treatment outcome of children with myeloid antigen positive acute lymphoblastic leukemia: a report from the Children's Cancer Group. Blood 90 (1): 28-35, 1997.
- Corrente F, Bellesi S, Metafuni E, et al.: Role of flow-cytometric immunophenotyping in prediction of BCR/ABL1 gene rearrangement in adult B-cell acute lymphoblastic leukemia. Cytometry B Clin Cytom 94 (3): 468-476, 2018.
- Hirabayashi S, Ohki K, Nakabayashi K, et al.: ZNF384-related fusion genes define a subgroup of childhood B-cell precursor acute lymphoblastic leukemia with a characteristic immunotype. Haematologica 102 (1): 118-129, 2017.
- Qian M, Zhang H, Kham SK, et al.: Whole-transcriptome sequencing identifies a distinct subtype of acute lymphoblastic leukemia with predominant genomic abnormalities of EP300 and CREBBP. Genome Res 27 (2): 185-195, 2017.
- Relling MV, Dervieux T: Pharmacogenetics and cancer therapy. Nat Rev Cancer 1 (2): 99-108, 2001.
- van Dongen JJ, Seriu T, Panzer-Grümayer ER, et al.: Prognostic value of minimal residual disease in acute lymphoblastic leukaemia in childhood. Lancet 352 (9142): 1731-8, 1998.
- Wood B, Wu D, Crossley B, et al.: Measurable residual disease detection by high-throughput sequencing improves risk stratification for pediatric B-ALL. Blood 131 (12): 1350-1359, 2018.
- Zhou J, Goldwasser MA, Li A, et al.: Quantitative analysis of minimal residual disease predicts relapse in children with B-lineage acute lymphoblastic leukemia in DFCI ALL Consortium Protocol 95-01. Blood 110 (5): 1607-11, 2007.
- Borowitz MJ, Devidas M, Hunger SP, et al.: Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children's Oncology Group study. Blood 111 (12): 5477-85, 2008.
- Borowitz MJ, Wood BL, Devidas M, et al.: Prognostic significance of minimal residual disease in high risk B-ALL: a report from Children's Oncology Group study AALL0232. Blood 126 (8): 964-71, 2015.
- Conter V, Bartram CR, Valsecchi MG, et al.: Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood 115 (16): 3206-14, 2010.
- O'Connor D, Enshaei A, Bartram J, et al.: Genotype-Specific Minimal Residual Disease Interpretation Improves Stratification in Pediatric Acute Lymphoblastic Leukemia. J Clin Oncol 36 (1): 34-43, 2018.
- Basso G, Veltroni M, Valsecchi MG, et al.: Risk of relapse of childhood acute lymphoblastic leukemia is predicted by flow cytometric measurement of residual disease on day 15 bone marrow. J Clin Oncol 27 (31): 5168-74, 2009.
- Pui CH, Pei D, Coustan-Smith E, et al.: Clinical utility of sequential minimal residual disease measurements in the context of risk-based therapy in childhood acute lymphoblastic leukaemia: a prospective study. Lancet Oncol 16 (4): 465-74, 2015.
- Rau RE, Dai Y, Devidas M, et al.: Prognostic impact of minimal residual disease at the end of consolidation in NCI standard-risk B-lymphoblastic leukemia: A report from the Children's Oncology Group. Pediatr Blood Cancer 68 (4): e28929, 2021.
- Schrappe M, Valsecchi MG, Bartram CR, et al.: Late MRD response determines relapse risk overall and in subsets of childhood T-cell ALL: results of the AIEOP-BFM-ALL 2000 study. Blood 118 (8): 2077-84, 2011.
- Teachey DT, Devidas M, Wood BL, et al.: Children's Oncology Group Trial AALL1231: A Phase III Clinical Trial Testing Bortezomib in Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia and Lymphoma. J Clin Oncol 40 (19): 2106-2118, 2022.
- Bartram J, Wade R, Vora A, et al.: Excellent outcome of minimal residual disease-defined low-risk patients is sustained with more than 10 years follow-up: results of UK paediatric acute lymphoblastic leukaemia trials 1997-2003. Arch Dis Child 101 (5): 449-54, 2016.
- Vora A, Goulden N, Mitchell C, et al.: Augmented post-remission therapy for a minimal residual disease-defined high-risk subgroup of children and young people with clinical standard-risk and intermediate-risk acute lymphoblastic leukaemia (UKALL 2003): a randomised controlled trial. Lancet Oncol 15 (8): 809-18, 2014.
- Pieters R, de Groot-Kruseman H, Van der Velden V, et al.: Successful Therapy Reduction and Intensification for Childhood Acute Lymphoblastic Leukemia Based on Minimal Residual Disease Monitoring: Study ALL10 From the Dutch Childhood Oncology Group. J Clin Oncol 34 (22): 2591-601, 2016.
- Gaynon PS, Desai AA, Bostrom BC, et al.: Early response to therapy and outcome in childhood acute lymphoblastic leukemia: a review. Cancer 80 (9): 1717-26, 1997.
- Borowitz MJ, Wood BL, Devidas M, et al.: Assessment of end induction minimal residual disease (MRD) in childhood B precursor acute lymphoblastic leukemia (ALL) to eliminate the need for day 14 marrow examination: A Children's Oncology Group study. [Abstract] J Clin Oncol 31 (Suppl 15): A-10001, 2013.
Also available online . Last accessed June 04, 2021. - Möricke A, Reiter A, Zimmermann M, et al.: Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood 111 (9): 4477-89, 2008.
- Griffin TC, Shuster JJ, Buchanan GR, et al.: Slow disappearance of peripheral blood blasts is an adverse prognostic factor in childhood T cell acute lymphoblastic leukemia: a Pediatric Oncology Group study. Leukemia 14 (5): 792-5, 2000.
- Volejnikova J, Mejstrikova E, Valova T, et al.: Minimal residual disease in peripheral blood at day 15 identifies a subgroup of childhood B-cell precursor acute lymphoblastic leukemia with superior prognosis. Haematologica 96 (12): 1815-21, 2011.
- Schrappe M, Hunger SP, Pui CH, et al.: Outcomes after induction failure in childhood acute lymphoblastic leukemia. N Engl J Med 366 (15): 1371-81, 2012.
- Möricke A, Zimmermann M, Valsecchi MG, et al.: Dexamethasone vs prednisone in induction treatment of pediatric ALL: results of the randomized trial AIEOP-BFM ALL 2000. Blood 127 (17): 2101-12, 2016.
- O'Connor D, Moorman AV, Wade R, et al.: Use of Minimal Residual Disease Assessment to Redefine Induction Failure in Pediatric Acute Lymphoblastic Leukemia. J Clin Oncol 35 (6): 660-667, 2017.
- Silverman LB, Gelber RD, Young ML, et al.: Induction failure in acute lymphoblastic leukemia of childhood. Cancer 85 (6): 1395-404, 1999.
- Oudot C, Auclerc MF, Levy V, et al.: Prognostic factors for leukemic induction failure in children with acute lymphoblastic leukemia and outcome after salvage therapy: the FRALLE 93 study. J Clin Oncol 26 (9): 1496-503, 2008.
- Schwab C, Ryan SL, Chilton L, et al.: EBF1-PDGFRB fusion in pediatric B-cell precursor acute lymphoblastic leukemia (BCP-ALL): genetic profile and clinical implications. Blood 127 (18): 2214-8, 2016.
- den Boer ML, Cario G, Moorman AV, et al.: Outcomes of paediatric patients with B-cell acute lymphocytic leukaemia with ABL-class fusion in the pre-tyrosine-kinase inhibitor era: a multicentre, retrospective, cohort study. Lancet Haematol 8 (1): e55-e66, 2021.
- Gupta S, Devidas M, Loh ML, et al.: Flow-cytometric vs. -morphologic assessment of remission in childhood acute lymphoblastic leukemia: a report from the Children's Oncology Group (COG). Leukemia 32 (6): 1370-1379, 2018.
- Moghrabi A, Levy DE, Asselin B, et al.: Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95-01 for children with acute lymphoblastic leukemia. Blood 109 (3): 896-904, 2007.
- Veerman AJ, Kamps WA, van den Berg H, et al.: Dexamethasone-based therapy for childhood acute lymphoblastic leukaemia: results of the prospective Dutch Childhood Oncology Group (DCOG) protocol ALL-9 (1997-2004). Lancet Oncol 10 (10): 957-66, 2009.
- Kosaka Y, Koh K, Kinukawa N, et al.: Infant acute lymphoblastic leukemia with MLL gene rearrangements: outcome following intensive chemotherapy and hematopoietic stem cell transplantation. Blood 104 (12): 3527-34, 2004.
- Balduzzi A, Valsecchi MG, Uderzo C, et al.: Chemotherapy versus allogeneic transplantation for very-high-risk childhood acute lymphoblastic leukaemia in first complete remission: comparison by genetic randomisation in an international prospective study. Lancet 366 (9486): 635-42, 2005 Aug 20-26.
- Schrauder A, Reiter A, Gadner H, et al.: Superiority of allogeneic hematopoietic stem-cell transplantation compared with chemotherapy alone in high-risk childhood T-cell acute lymphoblastic leukemia: results from ALL-BFM 90 and 95. J Clin Oncol 24 (36): 5742-9, 2006.
- Ribera JM, Ortega JJ, Oriol A, et al.: Comparison of intensive chemotherapy, allogeneic, or autologous stem-cell transplantation as postremission treatment for children with very high risk acute lymphoblastic leukemia: PETHEMA ALL-93 Trial. J Clin Oncol 25 (1): 16-24, 2007.
- Stanulla M, Dagdan E, Zaliova M, et al.: IKZF1plus Defines a New Minimal Residual Disease-Dependent Very-Poor Prognostic Profile in Pediatric B-Cell Precursor Acute Lymphoblastic Leukemia. J Clin Oncol 36 (12): 1240-1249, 2018.
Treatment Option Overview for Childhood ALL
Phases of Therapy
Treatment for children with acute lymphoblastic leukemia (ALL) is typically divided into the following phases:
- Remission induction chemotherapy (at the time of diagnosis).
- Postinduction therapy (after achieving complete remission).
- Consolidation/intensification therapy.
- Maintenance therapy.
Sanctuary Sites
Historically, certain extramedullary sites have been considered sanctuary sites (i.e., anatomic spaces that are poorly penetrated by many of the orally and intravenously administered chemotherapy agents typically used to treat ALL). The two most important sanctuary sites in childhood ALL are the central nervous system (CNS) and the testes. Successful treatment of ALL requires therapy that effectively addresses clinical or subclinical involvement of leukemia in these extramedullary sanctuary sites.
Central nervous system (CNS)
At diagnosis, approximately 3% of patients have CNS3 disease (defined as cerebrospinal fluid specimen with ≥5 white blood cells/μL with lymphoblasts and/or the presence of cranial nerve palsies). However, unless specific therapy is directed toward the CNS, most children will eventually develop overt CNS leukemia whether or not lymphoblasts were detected in the spinal fluid at initial diagnosis. CNS-directed treatments include intrathecal chemotherapy, CNS-directed systemic chemotherapy, and cranial radiation. Some or all of these treatments are included in current regimens for ALL. For more information, see the CNS-Directed Therapy for Childhood ALL section.
Testes
Overt testicular involvement at the time of diagnosis occurs in approximately 2% of males. In early ALL trials, testicular involvement at diagnosis was an adverse prognostic factor. With more aggressive initial therapy, however, the prognostic significance of initial testicular involvement is unclear.[
References:
- Hijiya N, Liu W, Sandlund JT, et al.: Overt testicular disease at diagnosis of childhood acute lymphoblastic leukemia: lack of therapeutic role of local irradiation. Leukemia 19 (8): 1399-403, 2005.
- Sirvent N, Suciu S, Bertrand Y, et al.: Overt testicular disease (OTD) at diagnosis is not associated with a poor prognosis in childhood acute lymphoblastic leukemia: results of the EORTC CLG Study 58881. Pediatr Blood Cancer 49 (3): 344-8, 2007.
Special Considerations for the Treatment of Children With ALL
The treatment of children and adolescents with acute lymphoblastic leukemia (ALL) entails complicated risk assignment, extensive therapies, and intensive supportive care (e.g., transfusions; management of infectious complications; and emotional, financial, and developmental support). Because of these factors, the evaluation and treatment of these patients are best coordinated by a multidisciplinary team in cancer centers or hospitals with all of the necessary pediatric supportive care facilities.[
- Primary care physicians.
- Pediatric medical oncologists and hematologists.
- Pediatric surgeons.
- Pathologists.
- Pediatric radiation oncologists.
- Pediatric intensivists.
- Rehabilitation specialists.
- Pediatric oncology nurses.
- Social workers.
- Child-life professionals.
- Psychologists.
- Nutritionists.
For specific information about supportive care for children and adolescents with cancer, see the summaries on
The American Academy of Pediatrics has outlined guidelines for pediatric cancer centers and their role in the treatment of children and adolescents with cancer.[
Clinical trials are generally available for children with ALL, with specific protocols designed for children at standard (low) risk of treatment failure and for children at higher risk of treatment failure. Clinical trials for children with ALL are generally designed to compare standard therapy for a particular risk group with a potentially better treatment approach that may improve survival and/or diminish toxicities associated with the standard treatment regimen. Other types of clinical trials test novel therapies when there is no standard therapy for a cancer diagnosis. Many of the therapeutic innovations that produced increased survival rates in children with ALL were achieved through clinical trials, and it is appropriate for children and adolescents with ALL to be offered participation in a clinical trial. Information about ongoing clinical trials is available from the
Risk-based treatment assignment is an important therapeutic strategy for children with ALL. This approach allows children who historically have a very good outcome to be treated with less intensive therapy and to be spared more toxic treatments, while children with a historically lower probability of long-term survival receive more intensive therapy that may increase their chance of cure. For more information about clinical and laboratory features that have shown prognostic value, see the Risk-Based Treatment Assignment section.
References:
- American Academy of Pediatrics: Standards for pediatric cancer centers. Pediatrics 134 (2): 410-4, 2014.
Also available online . Last accessed August 23, 2024. - Rubnitz JE, Lensing S, Zhou Y, et al.: Death during induction therapy and first remission of acute leukemia in childhood: the St. Jude experience. Cancer 101 (7): 1677-84, 2004.
- Christensen MS, Heyman M, Möttönen M, et al.: Treatment-related death in childhood acute lymphoblastic leukaemia in the Nordic countries: 1992-2001. Br J Haematol 131 (1): 50-8, 2005.
- Vrooman LM, Stevenson KE, Supko JG, et al.: Postinduction dexamethasone and individualized dosing of Escherichia Coli L-asparaginase each improve outcome of children and adolescents with newly diagnosed acute lymphoblastic leukemia: results from a randomized study--Dana-Farber Cancer Institute ALL Consortium Protocol 00-01. J Clin Oncol 31 (9): 1202-10, 2013.
- Lund B, Åsberg A, Heyman M, et al.: Risk factors for treatment related mortality in childhood acute lymphoblastic leukaemia. Pediatr Blood Cancer 56 (4): 551-9, 2011.
- Alvarez EM, Malogolowkin M, Li Q, et al.: Decreased Early Mortality in Young Adult Patients With Acute Lymphoblastic Leukemia Treated at Specialized Cancer Centers in California. J Oncol Pract 15 (4): e316-e327, 2019.
Treatment of Newly Diagnosed Childhood ALL
Standard Induction Treatment Options for Newly Diagnosed ALL
Standard treatment options for newly diagnosed childhood acute lymphoblastic leukemia (ALL) include the following:
- Chemotherapy.
Remission induction chemotherapy
The goal of the first phase of therapy (remission induction) is to induce a complete remission (CR). This induction phase typically lasts 4 weeks. Overall, approximately 98% of patients with newly diagnosed B-ALL achieve CR by the end of this phase, with somewhat lower rates in infants and in noninfant patients with T-ALL or high presenting leukocyte counts.[
Induction chemotherapy typically consists of the following drugs, with or without an anthracycline (either doxorubicin or daunorubicin):
- Vincristine.
- Corticosteroid (either prednisone or dexamethasone).
- Asparaginase.
- Intrathecal chemotherapy.
The Children's Oncology Group (COG) protocols administer a three-drug induction (vincristine, corticosteroid, and pegaspargase) to National Cancer Institute (NCI) standard-risk B-ALL patients and a four-drug induction (vincristine, corticosteroid, and pegaspargase plus anthracycline) to NCI high-risk B-ALL and all T-ALL patients. Other groups use a four-drug induction for all patients.[
Corticosteroid therapy
Many current regimens use dexamethasone instead of prednisone during remission induction and later phases of therapy, although controversy exists as to whether dexamethasone benefits all subsets of patients. Some trials also suggest that dexamethasone during induction may be associated with more toxicity than prednisone, including higher rates of infection, myopathy, and behavioral changes.[
Evidence (dexamethasone vs. prednisone during induction):
- The Children's Cancer Group conducted a randomized trial that compared dexamethasone and prednisone in standard-risk B-ALL patients receiving a three-drug induction without an anthracycline.[
6 ]- Dexamethasone was associated with a superior event-free survival (EFS).
- Dexamethasone was associated with a higher frequency of reversible steroid myopathy and hyperglycemia. No significant differences in rates of infection during induction were observed between the two randomized arms.
- Another randomized trial that included both standard-risk and high-risk patients was conducted by the United Kingdom Medical Research Council (MRC).[
7 ]- The trial demonstrated that dexamethasone was associated with a more favorable outcome than prednisolone in all patient subgroups.
- Patients who received dexamethasone had a significantly lower incidence of both central nervous system (CNS) and non-CNS relapses than did patients who received prednisolone.
- Dexamethasone was associated with a higher incidence of steroid-associated behavioral problems and myopathy, but an excess risk of osteonecrosis was not observed. There was no difference in induction death rates between the randomized groups.
- The Associazione Italiana di Ematologia e Oncologia Pediatrica (AIEOP) ALL-BFM-2000 (NCT00430118) trial randomly assigned 3,720 patients to receive either dexamethasone (10 mg/m2 /d) or prednisone (60 mg/m2 /d) during multiagent remission induction (including an anthracycline for all patients) after a 7-day prednisone prophase.[
9 ]- Dexamethasone was associated with higher incidence of life-threatening events (primarily infections), resulting in a significantly higher induction death rate (2.5% for dexamethasone vs. 0.9% for prednisone; P = .00013).
- There was no difference in rates of osteonecrosis between the randomized groups.
- The 5-year cumulative incidence of relapse was significantly lower with dexamethasone (11% vs. 16%; P < .0001), resulting in superior 5-year EFS rates (84% for dexamethasone vs. 81% for prednisone, P = .024) despite the increased induction death rate.
- No difference in overall survival (OS) was observed based on steroid randomization, although the study was not sufficiently powered to detect small differences in OS.
- In a predefined subgroup analysis, a survival benefit was observed with dexamethasone treatment in patients with T-ALL and a good response to the prednisone prophase (5-year OS rates, 91% with dexamethasone vs. 83% with prednisone, P = .036).
- The COG conducted a randomized trial of dexamethasone and prednisone in NCI high-risk B-ALL patients.[
8 ] Patients were randomly assigned to receive 14 days of dexamethasone or 28 days of prednisone during a four-drug induction (with an anthracycline). This trial also included a randomized comparison of high-dose and escalating-dose methotrexate during the interim maintenance phase.- Dexamethasone was associated with a higher rate of infection, but there was no difference in the induction death rate when comparing dexamethasone and prednisone.
- For patients who were younger than 10 years at diagnosis, there was a significant interaction between the corticosteroid and methotrexate randomizations. However, the best outcome for this group of patients was observed in those who received both dexamethasone during induction and high-dose methotrexate during interim maintenance.
- The corticosteroid randomization was closed early for patients aged 10 years or older at diagnosis because of excessive rates of osteonecrosis in patients randomly assigned to dexamethasone. However, there was no EFS benefit associated with dexamethasone in these older patients (5-year EFS rates of 73.1% with dexamethasone and 73.9% with prednisone; P = .78)
The ratio of dexamethasone to prednisone dose used may influence outcome. Studies in which the dexamethasone to prednisone ratio was 1:5 to 1:7 have shown a better result for dexamethasone, while studies that used a 1:10 ratio have shown similar outcomes.[
Asparaginase
Several forms of asparaginase have been used in the treatment of children with ALL, including the following:
- Pegaspargase (PEG-asparaginase).
- Calaspargase pegol.
- Asparaginase Erwinia chrysanthemi (Erwinia L-asparaginase).
- Native Escherichia coli (E. coli) L-asparaginase (unavailable in the United States, but still available in other countries).
Pegaspargase (PEG-asparaginase)
Pegaspargase is a form of L-asparaginase in which the E. coli–derived enzyme is modified by the covalent attachment of polyethylene glycol. It is commonly used during both induction and postinduction phases of treatment in newly diagnosed patients treated in Western Europe. Pegaspargase is not available in the United States, but it is still available in other countries.
Pegaspargase may be given either intramuscularly (IM) or intravenously (IV).[
Pegaspargase has a much longer serum half-life than native E. coli L-asparaginase, producing prolonged asparagine depletion after a single injection.[
Serum asparaginase enzyme activity levels of more than 0.1 IU/mL have been associated with serum asparagine depletion. Studies have shown that a single dose of pegaspargase given either IM or IV as part of multiagent induction results in serum enzyme activity of more than 0.1 IU/mL in nearly all patients for at least 2 to 3 weeks.[
In another study, doses of pegaspargase were reduced in an attempt to decrease toxicity.[
Evidence (use of pegaspargase versus native E. coli L-asparaginase):
- A randomized comparison of IV pegaspargase versus IM native E. coli asparaginase was conducted. Each agent was administered for a 30-week period after the achievement of CR.[
13 ][Level of evidence A3]- Serum asparaginase activity (SAA) levels were significantly higher with IV pegaspargase and exceeded goal therapeutic levels (>0.1 IU/mL) in nearly all patients throughout the 30-week period.
- There was no significant difference in EFS and OS between the randomized arms.
- There was no difference in rates of asparaginase-related toxicities, including hypersensitivity, pancreatitis, and thromboembolic complications.
- Similar outcome and similar rates of asparaginase-related toxicities were observed for both groups of patients.
- IV pegaspargase was associated with less treatment-related anxiety, as assessed by patient and parent surveys.
- Another randomized trial of patients with standard-risk ALL assigned patients to receive either pegaspargase or native E. coli asparaginase during induction and in each of two delayed intensification courses.[
15 ]- A single dose of pegaspargase given in conjunction with vincristine and prednisone during induction therapy appeared to have similar activity and toxicity as nine doses of IM E. coli L-asparaginase (3 times a week for 3 weeks).[
15 ] - The use of pegaspargase was associated with more rapid blast clearance and a lower incidence of neutralizing antibodies.
- A single dose of pegaspargase given in conjunction with vincristine and prednisone during induction therapy appeared to have similar activity and toxicity as nine doses of IM E. coli L-asparaginase (3 times a week for 3 weeks).[
Patients with an allergic reaction to pegaspargase are typically switched to Erwinia L-asparaginase. A COG analysis investigated the deleterious effect on disease-free survival (DFS) of early discontinuation of treatment with pegaspargase in patients with high-risk B-ALL. The study found that the adverse effect on outcome could be reversed with the use of Erwinia L-asparaginase to complete the planned course of asparaginase therapy.[
Evidence (adverse prognostic impact of early discontinuation of pegaspargase or silent inactivation of asparaginase):
- Several studies have identified a subset of patients who experience silent inactivation of asparaginase, which is defined as the absence of therapeutic SAA levels without overt allergy.[
23 ,24 ]- In a trial conducted by the Dana-Farber Cancer Institute (DFCI) Consortium, 12% of patients who were initially treated with native E.coli L-asparaginase demonstrated silent inactivation. These patients had a superior EFS if their asparaginase preparation was changed.[
24 ] - Patients who were treated with pegaspargase appear to have lower levels of silent inactivation (<10%).[
13 ,23 ,25 ]
Determination of the optimal frequency of pharmacokinetic monitoring for pegaspargase-treated patients, and whether such screening impacts outcome, awaits further investigation.
- In a trial conducted by the Dana-Farber Cancer Institute (DFCI) Consortium, 12% of patients who were initially treated with native E.coli L-asparaginase demonstrated silent inactivation. These patients had a superior EFS if their asparaginase preparation was changed.[
- A report from the COG included 8,196 patients with newly diagnosed B-ALL who were enrolled between 2004 to 2011.[
20 ][Level of evidence C2]- The cumulative incidence of pegaspargase discontinuation (because of toxicity) was 12.2% in NCI standard-risk patients and 25.4% in NCI high-risk patients.
- In multivariable analysis, NCI high-risk patients who discontinued pegaspargase early had inferior DFS (hazard ratio [HR], 1.5; P = .002) than did those who received all prescribed doses. For NCI standard-risk patients, there was no impact of pegaspargase discontinuation on DFS, except in patients with slow-early response who received intensified postinduction therapy (HR, 1.7; P = .03).
- NCI high-risk patients who discontinued pegaspargase but then switched to Erwinia asparaginase and received all subsequent intended doses, did not have an increased risk of relapse (HR, 1.1; P = .69).
- An analysis of 1,115 non–high-risk ALL patients from the Nordic Society of Pediatric Hematology and Oncology (NOPHO) ALL2008 protocol reported the following:[
25 ]- 255 patients received a truncated asparaginase course because of toxicity, and 46 patients had evidence of silent inactivation on therapeutic drug monitoring.
- The 7-year cumulative incidence of relapse was 11.1% in the 301 patients who received a truncated asparaginase course, compared with 6.7% in the remaining 814 patients who received the planned courses (HR, 1.73; P = .03).
- In a Cox model, suboptimal asparaginase treatment (because of either truncated pegaspargase or silent inactivation) was significantly associated with a higher relapse risk (HR, 1.69; P = 0.03).
In an attempt to decrease hypersensitivity reactions to pegaspargase, the Dutch Childhood Oncology Group-ALL11 protocol randomly assigned patients to receive either continuous or noncontinuous dosing after induction therapy. The occurrence of inactivating hypersensitivity reactions was seven times lower and antibody levels were significantly lower in the continuous-dosing arm. There was no difference in total number of asparaginase toxicities or the 5-year incidences of relapse, death, or disease-free survival between the treatment arms.[
Calaspargase pegol
Calaspargase pegol is another formulation of pegylated asparaginase that is also available for the treatment of children and adolescents with ALL.[
Evidence (calaspargase pegol vs. pegaspargase):
- In a COG study, 165 patients with high-risk B-ALL were randomly assigned to receive either calaspargase pegol or pegaspargase during the induction phase of ALL therapy.[
28 ]- The mean half-life of calaspargase pegol was approximately 2.5 times longer than pegaspargase.
- The total systemic exposure to calaspargase pegol was greater than for pegaspargase.
- Twenty-five days after a dose of calaspargase pegol, 95% of patients maintained an asparaginase level higher than 0.1 IU/mL, compared with 28% of patients who received pegaspargase.
- Evidence of end-induction minimal residual disease (MRD) negativity was similar between the two drugs (74% and 72%).
- The toxicity profile of the two drugs was similar.
- In a DFCI trial of calaspargase pegol in patients with newly diagnosed ALL, all patients received one dose of either calaspargase pegol or pegaspargase as part of induction therapy. After induction, 230 patients were randomly assigned to receive either calaspargase pegol every 3 weeks (10 doses) or pegaspargase every 2 weeks (15 doses).[
29 ]- At day 25 after the induction dose, 88% of patients who received calaspargase pegol had an asparaginase level higher than 0.1 IU/mL, compared with 17% of patients who received pegaspargase.
- There was no difference in end-of-induction MRD.
- There was no difference in the frequency of toxicities (37%).
- The 5-year EFS rates were similar for calaspargase pegol and pegaspargase (88.1% vs. 84.9%).
Calaspargase pegol has only been approved for use in the United States for patients younger than 22 years.
AsparaginaseErwinia chrysanthemi(ErwiniaL-asparaginase)
Erwinia L-asparaginase is typically used in patients who have experienced an allergy to native E. coli or pegaspargase.
The half-life of Erwinia L-asparaginase (0.65 days) is much shorter than that of native E. coli (1.2 days) or pegaspargase (5.7 days).[
Evidence (increased dose frequency of Erwinia L-asparaginase needed to achieve goal therapeutic effect):
- A COG trial demonstrated that IM Erwinia L-asparaginase given three times a week to patients with an allergy to pegaspargase leads to therapeutic serum asparaginase enzyme activity levels (defined as a level ≥0.1 IU/mL).[
30 ]- On this trial, 96% of children achieved a level of 0.1 IU/mL or more at 2 days after a dose of Erwinia L-asparaginase and 85% did so at 3 days after a dose.
- A trial of IV Erwinia L-asparaginase given on a Monday-Wednesday-Friday schedule to patients with an allergy to pegaspargase demonstrated therapeutic serum asparaginase enzyme activity (defined as ≥0.1 IU/mL) in 83% of patients 48 hours after a dose but in only 43% of patients 72 hours after a dose.[
31 ]- If IV Erwinia is given on a Monday-Wednesday-Friday schedule, the authors suggest that 72-hour nadir enzyme activity levels be monitored to ensure therapeutic levels.
A recombinant form of Erwinia L-asparaginase, asparaginase erwinia chrysanthemi (recombinant)-rywn, was studied in a phase II/III COG trial. When it was given on a Monday (25 mg/m2), Wednesday (25 mg/m2), and Friday (50 mg/m2) schedule for six doses, the proportion of patients who achieved asparaginase levels of 0.1 IU/mL or greater was 90% at 72 hours (44 of 49 patients) and 96% at 48 hours (47 of 49 patients). The safety profile was comparable with other forms of asparaginase.[
Anthracycline use during induction
The COG protocols administer a three-drug induction (vincristine, corticosteroid, and pegaspargase) to NCI standard-risk B-ALL patients and a four-drug induction (vincristine, corticosteroid, and pegaspargase plus an anthracycline) to NCI high-risk B-ALL and all T-ALL patients. Other groups use a four-drug induction for all patients.[
In induction regimens that include an anthracycline, either daunorubicin or doxorubicin are typically used. In a randomized trial comparing the two agents during induction, there were no differences in early response measures, including reduction in peripheral blood blast counts during the first week of therapy, day 15 marrow morphology, and end-induction MRD levels.[
Response to remission induction chemotherapy
More than 95% of children with newly diagnosed ALL will achieve a CR within the first 4 weeks of treatment. Of those who fail to achieve CR within the first 4 weeks, approximately one-half will experience a toxic death during the induction phase (usually caused by infection) and the other half will have resistant disease (persistent morphological leukemia).[
Remission is classically defined as an end-induction bone marrow examination by routine microscopic cytomorphology with fewer than 5% lymphoblasts at the end of induction (M1). The Ponte de Legno consortium includes approximately 15 large national and international cooperative groups devoted to the study and treatment of childhood ALL. This group published a consensus definition of complete remission, as follows:[
- Achievement of MRD levels of less than 1% and/or M1 cytomorphology.
- MRD is the gold standard and takes precedence over cytomorphology.
- MRD is determined by either flow cytometry or polymerase chain reaction techniques.
- Resolution of extramedullary disease, assessed no earlier than the end of induction.
Most patients with persistence of morphologically detectable leukemia at the end of the 4-week induction phase have a poor prognosis and may benefit from an allogeneic hematopoietic stem cell transplant (HSCT) once CR is achieved.[
A follow-up retrospective study reported the outcomes of 325 children and adolescents with T-ALL and initial induction failure who were treated between 2000 and 2018.[
The incorporation of nelarabine may be of value for patients with T-ALL and have induction failure. The COG AALL0434 (NCT00408005) study included 43 patients with more than 25% blasts in an end-induction bone marrow aspirate. Of these patients, 23 patients were nonrandomly assigned to therapy that included high-dose methotrexate and nelarabine as part of a multidrug regimen, and 20 patients underwent allogeneic transplant. The 5-year EFS rate was 53.1% (± 9.4%) for the patients who received high-dose methotrexate and nelarabine. There was no difference in outcome for these two groups (HR, 0.66; 95% CI, 0.24–1.83; P = .423).[
For patients who achieve CR, measures of the rapidity of blast clearance and MRD determinations have important prognostic significance, particularly the following:
- The percentage of morphologically detectable marrow blasts at 7 and 14 days after starting multiagent remission induction therapy has been correlated with relapse risk,[
44 ] and has been used in the past by the COG to risk-stratify patients. However, in multivariate analyses, when end-induction MRD is included, these early marrow findings lose their prognostic significance.[45 ,46 ] - End-induction levels of submicroscopic MRD, assessed by multiparameter flow cytometry, polymerase chain reaction, or next-generation sequencing assays strongly correlates with long-term outcome.[
45 ,47 ,48 ,49 ,50 ] Intensification of postinduction therapy for patients with high levels of end-induction MRD is a common component of most ALL treatment regimens. In a randomized trial conducted by the United Kingdom Acute Lymphoblastic Leukaemia (UKALL) group, augmented postinduction therapy was shown to improve outcome for standard-risk and intermediate-risk patients with high end-induction MRD.[51 ] - MRD levels earlier in induction (e.g., days 8 and 15) and at later postinduction time points (e.g., week 12 after starting therapy) have also been shown to have prognostic significance in both B-ALL and T-ALL.[
45 ,46 ,49 ,52 ,53 ,54 ,55 ] - Nearly all patients with a positive end-of-induction MRD will become MRD negative at the end of 4 to 8 weeks of consolidation therapy. In a COG study, patients with high-risk B-ALL who had a positive end-of-induction MRD but a negative end-of-consolidation MRD had a significantly improved DFS compared with patients who were MRD positive at end of consolidation (5-year DFS rate, 79.5% vs. 39.5%).[
46 ]
For more information, see the Response to initial treatment section.
For specific information about CNS therapy to prevent CNS relapse in children with newly diagnosed ALL, see the CNS-Directed Therapy for Childhood ALL section.
Standard Postinduction Treatment Options for Childhood ALL
Standard treatment options for consolidation/intensification and maintenance therapy (postinduction therapy) include the following:
- Chemotherapy.
CNS-directed therapy is provided during premaintenance chemotherapy by all groups. Some protocols (COG, St. Jude Children's Research Hospital [SJCRH], and DFCI) provide ongoing intrathecal chemotherapy during maintenance, while others (Berlin-Frankfurt-Münster [BFM]) do not. For specific information about CNS therapy to prevent CNS relapse in children with acute lymphoblastic leukemia (ALL) who are receiving postinduction therapy, see the CNS-Directed Therapy for Childhood ALL section.
Consolidation/intensification therapy
Once CR has been achieved, systemic treatment in conjunction with CNS-directed therapy follows. The intensity of the postinduction chemotherapy varies considerably depending on risk group assignment, but all patients receive some form of intensification after the achievement of CR and before beginning maintenance therapy.
The most commonly used intensification schema is the BFM backbone. This therapeutic backbone, first introduced by the BFM clinical trials group, includes the following:[
- An initial consolidation (referred to as induction IB) immediately after the initial induction phase. This phase includes intrathecal therapy, cyclophosphamide, low-dose cytarabine, and mercaptopurine.
An interim maintenance phase, which includes intrathecal therapy and four doses of high-dose methotrexate (typically 5 g/m2) with leucovorin rescue.
- Reinduction (or delayed intensification), which typically includes agents and schedules similar to those used during the induction and initial consolidation phases.
- Maintenance, typically consisting of daily mercaptopurine (6-MP), weekly low-dose methotrexate, and sometimes, intermittent administration of vincristine and a corticosteroid, as well as continued intrathecal therapy.
This backbone has been adopted by many groups, including the COG. Variation of this backbone includes the following:
- Intensification for higher-risk patients by including additional doses of vincristine and pegaspargase, as well as repeated interim maintenance and delayed intensification phases.[
56 ,57 ] - The use of escalating doses of methotrexate (starting at a dose of 100 mg/m2) without leucovorin rescue instead of or in addition to high-dose methotrexate during interim maintenance phases.
- Elimination or truncation of some of the phases for lower-risk patients to minimize acute and long-term toxicity.
Other clinical trial groups utilize a different therapeutic backbone during postinduction treatment phases, as follows:
- DFCI: The DFCI ALL Consortium protocols include 30 weeks of pegaspargase therapy beginning at week 7 of therapy, given in conjunction with maintenance regimen (vincristine/dexamethasone pulses, weekly low-dose methotrexate, daily mercaptopurine).[
3 ] These protocols also do not include a delayed intensification phase, but high-risk patients receive additional doses of doxorubicin (instead of low-dose methotrexate) during the first six months of postinduction therapy. - NOPHO: The NOPHO also emphasizes the use of pegaspargase during consolidation and intensification. In the ALL2008 trial, all patients received five doses of pegaspargase given every other week after induction. Patients then received an additional ten doses at 2-week intervals or three doses at 6-week intervals. Both regimens produced equally excellent survival rates, with reduced toxicity in the three-dose regimen.[
58 ] - SJCRH: SJCRH follows a BFM backbone but augments the reinduction and maintenance phases for some patients by including intensified dosing of pegaspargase, frequent vincristine/corticosteroid pulses, and rotating drug pairs during maintenance (mercaptopurine/methotrexate, cyclophosphamide/cytarabine, dexamethasone/vincristine).[
59 ]
Standard-risk ALL
In children with low- and standard-risk B-ALL, there has been an attempt to limit exposure to drugs such as anthracyclines and alkylating agents that may be associated with an increased risk of late toxic effects.[
Favorable outcomes for standard-risk patients with B-ALL were also reported in trials that used a limited number of courses of intermediate-dose or high-dose methotrexate as consolidation followed by maintenance therapy (without a reinduction phase).[
However, the prognostic impact of end-induction and/or consolidation MRD has influenced the treatment of patients originally diagnosed as NCI standard risk. Multiple studies have demonstrated that higher levels of end-induction MRD are associated with poorer prognosis.[
Evidence (intensification for standard-risk B-ALL):
- Clinical trials conducted in the 1980s and early 1990s demonstrated that the use of a delayed intensification phase improved outcome for children with standard-risk ALL treated with regimens using a BFM backbone.[
71 ,72 ,73 ] The delayed intensification phase on such regimens, including those of the COG, consists of an 8-week phase of reinduction (including dexamethasone and an anthracycline) and reconsolidation containing cyclophosphamide, cytarabine, and 6-thioguanine given approximately 4 to 6 months after remission is achieved.[35 ,71 ,74 ] - The former Children's Cancer Group (CCG) study (CCG-1991/COG-1991) for standard-risk ALL used dexamethasone in a three-drug induction phase and tested the utility of a second delayed intensification phase. This study also compared escalating IV methotrexate (without leucovorin rescue) in conjunction with vincristine versus a standard maintenance combination with oral methotrexate given during two interim maintenance phases.[
75 ][Level of evidence B1]- A second delayed intensification phase provided no benefit in patients who were rapid early responders (M1 or M2 marrow by day 14 of induction).
- Escalating IV methotrexate during the interim maintenance phases, compared with oral methotrexate during these phases, produced a significant improvement in EFS, which was because of a decreased incidence of isolated extramedullary relapses, particularly those involving the CNS.
- Successful therapies for patients with standard-risk ALL that have decreased the use of drugs associated with long-term toxicities have focused on children with B-ALL, not T-ALL.[
61 ,62 ,64 ,65 ,67 ,68 ] The COG, Dutch Children's Oncology Group (DCOG), DFCI, NOPHO, and other large cooperative groups have excluded patients with T-cell ALL from low and standard-risk therapies. Patients with NCI standard-risk features but a T-cell immunophenotype had an inferior EFS and OS compared with patients with NCI standard-risk B-ALL treated on the same regimens on CCG1952 and CCG1991.[76 ]
- The COG AALL0331 (NCT00103285) study stratified intensity of therapy for NCI standard-risk patients on the basis of biology and early response. Rapid early response was defined as less than 5% bone marrow blasts by day 15 based on local morphological interpretation and an M1 bone marrow with MRD levels of less than 0.1% at day 29. Standard-risk low patients were those with favorable biology (ETV6::RUNX1 or high hyperdiploidy with triple trisomy), CNS1 status, and a rapid early response. Standard-risk average patients were those lacking favorable or unfavorable biology who also had a rapid early response. Standard-risk high patients were those with slow early response and/or CNS3 status, or KMT2A-rearranged patients with rapid early response. All patients received a three-drug prednisone-based induction (no anthracycline). Standard-risk average patients were randomly assigned to either intensified consolidation (augmented BFM) or standard consolidation. Standard-risk high patients were nonrandomly assigned to the full augmented BFM therapy used for NCI high-risk patients, including two delayed intensification phases.[
77 ]- The 6-year EFS rate for all patients was 89%, and the OS rate was 96%.
- For standard-risk low patients, this study evaluated the addition of four doses of pegaspargase (added in consolidation and interim maintenance phases) to standard therapy, which included two doses of pegaspargase (administered in induction and delayed intensification phases). Standard-risk low patients had highly favorable outcomes (6-year DFS and OS rates were 94.7% ± 0.6% and 98.7% ± 0.3%, respectively). The augmentation of standard-risk low therapy with additional pegaspargase did not improve outcomes.[
63 ][Level of evidence B4] - For standard-risk average patients, the augmented consolidation regimen did not improve rates of continuous complete remission (CCR) or OS. The 6-year rates of CCR and OS for the standard-risk average cohort were 88% to 89% and 95% to 96%, respectively.
- Standard-risk average patients with end-induction MRD levels of 0.01% to <0.1% had an inferior outcome compared with those with MRD levels of <0.01% (6-year CCR rates, 77% vs. 91%, respectively). Augmented consolidation was not associated with a better outcome in standard-risk average patients with higher levels of MRD.
- The standard-risk high cohort achieved a relatively favorable 6-year CCR rate of 86% and an OS rate of 93%.
- In a randomized study conducted in the United Kingdom, children and young adults with ALL who lacked high-risk features (including adverse cytogenetics, and/or M3 marrow morphology at day 8 or day 15 of induction) were risk-stratified on the basis of MRD level at the end of induction (week 4) and at week 11 of therapy. Patients with undetectable MRD at week 4 (or with low MRD at week 4 and undetectable by week 11) were considered low risk, and were eligible to be randomly assigned to therapy with either one or two delayed intensification phases.[
78 ][Level of evidence B1]- There was no significant difference in EFS between patients who received one and those who received two delayed intensification phases.
- There was no significant difference in treatment-related deaths between the two arms; however, the second delayed intensification phase was associated with grade 3 or 4 toxic events in 17% of the 261 patients randomly assigned to that arm, and one patient experienced a treatment-related death during that phase.
- In the AIEOP ALL-BFM-2000 (NCT00430118) trial, standard-risk patients (defined as those with undetectable MRD at days 33 and 78 and absence of high-risk cytogenetics) were randomly assigned to receive treatment with a single delayed-intensification phase of either standard intensity or reduced intensity (shorter duration, with reduced total dosages of dexamethasone, vincristine, doxorubicin, and cyclophosphamide).[
79 ]- Reduced-intensity delayed intensification was associated with an inferior 8-year DFS rate (89% vs. 92%, P = .04), resulting from an increased risk of relapse.
- In a subset analysis, for patients with the ETV6::RUNX1 fusion, no difference in outcome between the two treatment arms was observed (8-year DFS rate, approximately 94% for both arms).
- The Malaysia-Singapore ALL MS2010 trial for patients with favorable-risk B-ALL evaluated a deintensified, modified BFM regimen. This regimen omitted anthracyclines, had fewer doses of high-dose methotrexate, and fewer doses of low-dose cytarabine.[
80 ]- The long-term EFS in this trial (6-year EFS rate, 96.5%) was noninferior, compared with the predecessor trial conducted by the same group.
- This regimen was also less toxic, with significantly decreased rates of bacteremia and septic shock/intensive care unit admissions.
- Patients who are standard or intermediate risk at diagnosis, but have high levels of end-induction MRD, have been shown to have a poorer prognosis and should be treated as high-risk patients. The UKALL2003 (NCT00222612) trial used augmented postinduction therapy (extra doses of pegaspargase and vincristine and an escalated-dose of IV methotrexate without leucovorin rescue) to treat standard- or intermediate-risk patients with high levels of end-induction MRD.[
51 ][Level of evidence B1]- Augmented postinduction therapy resulted in an increased EFS that was comparable to that of patients with low levels of end-induction MRD.
High-risk ALL
In high-risk patients, a number of different approaches have been used with comparable efficacy.[
Evidence (intensification for high-risk ALL):
- The former CCG developed an augmented BFM treatment regimen that included a second interim maintenance and delayed intensification phase. This regimen featured repeated courses of escalating-dose IV methotrexate (without leucovorin rescue) given with vincristine and pegaspargase during interim maintenance and additional vincristine and pegaspargase pulses during initial consolidation and delayed intensification. In the CCG-1882 trial, NCI high-risk patients with slow early response (M3 marrow on day 7 of induction) were randomly assigned to receive either standard- or augmented-BFM therapy.[
56 ]- The augmented-therapy regimen in the CCG-1882 trial produced significantly better EFS and OS rates (75% and 78%) than did the standard CCG modified-BFM therapy (55% and 66.7%).
- There was a significantly higher incidence of osteonecrosis in patients older than 10 years who received the augmented therapy (which included two 21-day postinduction dexamethasone courses), compared with those who were treated on the standard arm (one 21-day postinduction dexamethasone course).[
82 ]
- In an Italian study, investigators showed that two applications of delayed intensification therapy (protocol II) significantly improved outcome for patients with a poor response to a prednisone prophase.[
83 ] - The CCG-1961 study used a 2 × 2 factorial design to compare both standard- versus augmented-intensity therapies and therapies of standard duration (one interim maintenance and delayed intensification phase) versus increased duration (two interim maintenance and delayed intensification phases) among NCI high-risk patients with a rapid early response. This trial also tested whether continuous versus alternate-week dexamethasone during delayed intensification phases affected rates of osteonecrosis.
- Augmented therapy was associated with an improvement in EFS. There was no EFS benefit associated with the administration of the second interim maintenance and delayed intensification phases.[
57 ,84 ][Level of evidence A1] - The cumulative incidence of osteonecrosis at 5 years was 9.9% for patients aged 10 to 15 years and 20.0% for patients aged 16 to 21 years, compared with 1.0% for patients aged 1 to 9 years (P = .0001). For patients aged 10 to 21 years, alternate-week dosing of dexamethasone during delayed intensification phases was associated with a significantly lower cumulative incidence of osteonecrosis, compared with continuous dosing (8.7% vs. 17.0%, P = .0005).[
85 ][Level of evidence A3]
- Augmented therapy was associated with an improvement in EFS. There was no EFS benefit associated with the administration of the second interim maintenance and delayed intensification phases.[
- In the UKALL2003 (NCT00222612) trial, patients with high end-induction MRD (>0.01%) and/or high-risk cytogenetics were randomly assigned to receive either a standard-intensity or an augmented BFM chemotherapy backbone.[
86 ]- The 10-year EFS rate was 87.1% for patients assigned to the augmented chemotherapy backbone, compared with 82.1% for those assigned the standard-intensity chemotherapy backbone (P = .09).
- Patients with high-risk cytogenetics had a significantly lower risk of relapse when treated with augmented therapy (10-year relapse rate, 22.1% vs. 52.4% with standard-intensity therapy; P = .016).
- In the COG AALL0232 (NCT00075725) study (2004–2011), patients with high-risk B-ALL received an augmented BFM backbone with one interim maintenance and delayed intensification phase. Only patients with end-induction MRD greater than 0.1% or M2/M3 marrow at day 15 received two interim maintenance/delayed intensification phases. Patients were randomly assigned to receive either high-dose methotrexate or escalating dose IV methotrexate (Capizzi methotrexate) plus pegaspargase during the interim maintenance phase (the first phase only for those receiving two of these phases).[
8 ,46 ]- The methotrexate randomization was terminated early when planned interim monitoring indicated that high-dose methotrexate was associated with superior outcome. The 5-year EFS rate of patients randomly assigned to high-dose methotrexate was 79.6%, compared with 75% for those randomly assigned to the Capizzi methotrexate arm. High-dose methotrexate was also associated with a superior 5-year OS (P = .025).[
8 ] - Patients with MRD less than 0.01% at end of induction had a 5-year EFS rate of 87%, compared with 74% for those with MRD 0.01% to 0.1%. Those with MRD levels greater than 0.1% fared worse.[
46 ] - High-dose methotrexate was associated with a superior EFS rate in patients with end-induction MRD greater than 0.01% (high-dose methotrexate, 68%; Capizzi methotrexate, 58%; P = .008).[
46 ]
- The methotrexate randomization was terminated early when planned interim monitoring indicated that high-dose methotrexate was associated with superior outcome. The 5-year EFS rate of patients randomly assigned to high-dose methotrexate was 79.6%, compared with 75% for those randomly assigned to the Capizzi methotrexate arm. High-dose methotrexate was also associated with a superior 5-year OS (P = .025).[
Because treatment for high-risk ALL involves more intensive therapy, leading to a higher risk of acute and long-term toxicities, a number of clinical trials have tested interventions to prevent side effects without adversely impacting EFS. Interventions that have been investigated include the use of the cardioprotectant dexrazoxane to prevent anthracycline-related cardiac toxic effects and alternative scheduling of corticosteroids to reduce the risk of osteonecrosis.
Evidence (cardioprotective effect of dexrazoxane):
- In a DFCI ALL Consortium trial, children with high-risk ALL were randomly assigned to receive doxorubicin alone (30 mg/m2 /dose to a cumulative dose of 300 mg/m2) or with dexrazoxane during the induction and intensification phases of multiagent chemotherapy.[
87 ,88 ]- The use of the cardioprotectant dexrazoxane before doxorubicin resulted in better left ventricular fractional shortening and improved end-systolic dimension Z-scores without any adverse effect on EFS or increase in second malignancy risk, compared with the use of doxorubicin alone 5 years posttreatment.
- A greater long-term protective effect was noted in girls than in boys.
- On the POG-9404 trial, patients with T-ALL were randomly assigned to receive dexrazoxane or not before each dose of doxorubicin (cumulative dose 360 mg/m2).[
89 ]- There was no difference in EFS between patients with T-ALL who were treated with dexrazoxane and patients who were not treated with dexrazoxane (cumulative doxorubicin dose, 360 mg/m2).
- Three years after initial diagnosis, left ventricular shortening fraction and left ventricular wall thickness were both significantly worse in patients who received doxorubicin alone than in patients who received dexrazoxane, indicating that dexrazoxane was cardioprotective. The frequency of grade 3 and 4 toxicities that occurred during therapy was similar between the randomized groups, and there was no difference in cumulative incidence of second malignant neoplasms.
Evidence (reducing risk of osteonecrosis):
- In the CCG-1961 study, alternate-week dosing of dexamethasone during delayed intensification was studied with the goal of reducing the frequency of osteonecrosis.[
85 ] Patients with high-risk B-ALL and a rapid early morphological response to induction therapy were randomly assigned to receive either one or two delayed intensification phases. Patients randomly assigned to one delayed intensification phase received daily dosing of dexamethasone (21 consecutive days), while those randomly assigned to two delayed intensification phases received alternate-week dosing of dexamethasone (days 0–6 and 14–21) during each delayed intensification phase.- For patients aged 10 years or older at diagnosis, those who received two delayed intensification phases (alternate-week dosing of dexamethasone) had a significantly lower risk of symptomatic osteonecrosis (5-year cumulative incidence of 8.7%, compared with 17% for patients receiving one delayed intensification phase with continuous dexamethasone dosing; P = .001).
- The greatest impact was seen in females aged 16 to 21 years, who showed the highest incidence of osteonecrosis with standard therapy containing continuous dexamethasone; the incidence of osteonecrosis with alternative-week dexamethasone was 5.6%, compared with 57.6% for those receiving continuous dosing.
For more information, see the Osteonecrosis section.
Very high-risk ALL
Approximately 10% to 20% of patients with ALL are classified as very high risk, including the following:[
- Infants younger than 1 year, especially if there is a KMT2A gene rearrangement present. For more information about infants with ALL, see the Infants With ALL section.
- Patients with adverse cytogenetic abnormalities, including BCR::ABL1, TCF3::HLF, KMT2A gene rearrangements, and low hypodiploidy (<44 chromosomes).
- Patients who achieve CR but have a slow early response to initial therapy, including those with a high absolute blast count after a 7-day steroid prophase, and patients with high MRD levels at the end of induction (week 4) or later time points (e.g., week 12).
- Patients who have morphologically persistent disease after the first 4 weeks of therapy (induction failure), even if they later achieve CR.
Patients with very high-risk features have been treated with multiple cycles of intensive chemotherapy during the consolidation phase (usually in addition to the typical BFM backbone intensification phases). These additional cycles often include agents not typically used in frontline ALL regimens for standard-risk and high-risk patients, such as high-dose cytarabine, ifosfamide, and etoposide.[
On some clinical trials, very high-risk patients have also been considered candidates for allogeneic HSCT in first CR.[
Evidence (allogeneic HSCT in first remission for very high-risk patients):
- In a European cooperative group study conducted between 1995 and 2000, very high-risk patients were defined as one of the following: morphologically persistent disease after a four-drug induction, BCR::ABL1 or KMT2A::AFF1 fusions, or poor response to prednisone prophase in patients with either T-cell phenotype or presenting white blood cells (WBC) >100,000/μL. These patients were assigned to receive either an allogeneic HSCT in first CR (based on the availability of a human lymphocyte antigen–matched related donor) or intensive chemotherapy.[
39 ]- Using an intent-to-treat analysis, patients assigned to allogeneic HSCT (on the basis of donor availability) had a superior 5-year DFS rate compared with patients assigned to intensive chemotherapy (57% ± 7% for transplant vs. 41% ± 3% for chemotherapy, P = .02).
- There was no significant difference in OS rates (56% ± 6% for transplant vs. 50% ± 3% for chemotherapy; P = .12).
- For patients with T-ALL and a poor response to prednisone prophase, both DFS and OS rates were significantly better with allogeneic HSCT.[
92 ]
- In a large retrospective series of patients with initial induction failure, the 10-year OS rate for patients with persistent leukemia was 32%.[
41 ]- A trend for superior outcome with allogeneic HSCT, compared with chemotherapy alone, was observed in patients with T-cell phenotype (any age) and with B-ALL who were older than 6 years.
- Patients with B-ALL who were aged 1 to 5 years at diagnosis and did not have any adverse cytogenetic abnormalities (KMT2A rearrangement, BCR::ABL1) had a relatively favorable prognosis, without any advantage in outcome with the utilization of HSCT compared with chemotherapy alone.
- The AIEOP ALL-BFM-2000 (NCT00430118) study (2000–2006) classified patients as high risk if they met any of the following criteria: poor response to prednisone prophase, failure to achieve CR at the end of the first month of treatment, high MRD levels after induction IB (day 78 of therapy), and KMT2A::AFF1 fusion. These patients were allocated to allogeneic HSCT in first CR per protocol on the basis of donor availability and investigator preference.[
95 ][Level of evidence B4]- The overall 5-year EFS rate of patients meeting high-risk criteria was 58.9%.
- The 5-year EFS rate was 74% for patients whose only high-risk feature was prednisone-poor response. There was no significant difference in DFS (P = .31) or OS (P = .91) when comparing HSCT and chemotherapy for patients with poor prednisone response in whom HSCT was allowed per protocol (those with T-ALL and/or WBC ≥100,000/mm3).
- All other high-risk patients (i.e., those with initial induction failure, high day 78 MRD and/or KMT2A::AFF1 fusion) had EFS rates less than 50%. For these patients, there was no statistically significant difference in DFS between those who received HSCT (n = 66) and those who received chemotherapy only (n = 88), after adjusting for waiting time to HSCT (5.7 months).
- On the NOPHO ALL2008 (NCT00819351) protocol, patients were allocated to HSCT in first CR if they had MRD levels of 5% or greater at the end of induction or MRD levels of 0.1% or greater at end of consolidation. All patients allocated to HSCT received at least three blocks of intensive chemotherapy before HSCT to reduce levels of MRD.[
96 ]- In the intent-to-treat analysis of 69 patients who met HSCT criteria (10 of whom did not undergo HSCT), the 5-year DFS rate was 78%.
- Comparing the patients in this cohort who did and did not receive HSCT, receipt of HSCT was not significantly associated with survival (HR, 1.4; P = .69).
- For patients who underwent HSCT, superior outcomes (better DFS and lower cumulative incidence of relapse) were observed in patients who had nondetectable MRD before HSCT.
- In the DCOG ALL-10 and ALL-11 trials, patients with very high-risk features received an intensified treatment regimen that included three high-dose chemotherapy blocks after consolidation. After the three blocks, 60 patients received an allogeneic HSCT, and 22 patients continued with a chemotherapy-only approach, which included three additional high-dose blocks. In these trials, very high-risk disease was defined by any of the following features: morphologically detectable disease at end of induction, high end-consolidation MRD (time point 2), t(4;11), or poor response to a prednisone prophase.[
91 ]- The 5-year EFS rate was 72.8% for all patients.
- In a landmark analysis of EFS from the end of the third high-dose chemotherapy block, no difference was observed in the outcomes of patients who received HSCT versus those who received chemotherapy only.
- Two retrospective analyses investigated the role of HSCT in first CR for patients with hypodiploid ALL. The studies showed no clear evidence that HSCT improved outcomes when 1) transplanting all patients with hypodiploid ALL, or 2) transplanting hypodiploid patients deemed at high risk on the basis of high MRD after induction. The studies did not examine the strategy of HSCT for persistent MRD after consolidation, nor did they analyze the status of MRD at the time of HSCT.
- In a study of 306 hypodiploid patients from 16 ALL cooperative groups treated between 1997 and 2013, a subgroup of 228 patients (42 who underwent HSCT) with 44 or fewer chromosomes who achieved remission were analyzed.[
97 ][Level of evidence C2]- Favorable prognostic factors included a chromosome number of 44 (compared with 43 or fewer), MRD less than 0.01% after induction, and treatment on an MRD-stratified protocol that intensified therapy for patients with higher MRD after induction.
- After correction for median time to transplant, patients with low MRD who underwent HSCT had a DFS rate of 73.6%, compared with a DFS rate of 70% for those treated with chemotherapy alone (P = .81). Patients with higher MRD after induction who underwent HSCT had a DFS rate of 55.9%, compared with a DFS rate of 40.3% for those treated with chemotherapy (P = .29).
- The COG published an analysis of 113 evaluable patients with hypodiploid ALL who were treated between 2003 and 2011; 61 of those patients underwent HSCT in first CR.[
98 ][Level of evidence C1]- The 5-year EFS rate was 57.4% for patients who underwent HSCT and 47.8% for patients in the chemotherapy cohorts (P = .49). The OS rate was 66.2% for patients who underwent HSCT and 53.8% for patients in the chemotherapy cohorts (P = .34).
- Patients with high MRD after induction (≥0.01%) had a very poor EFS rate of 26.7% at 5 years, with no difference between the patients who received HSCT and the patients who received chemotherapy.
- In a study of 306 hypodiploid patients from 16 ALL cooperative groups treated between 1997 and 2013, a subgroup of 228 patients (42 who underwent HSCT) with 44 or fewer chromosomes who achieved remission were analyzed.[
Maintenance therapy
Backbone of maintenance therapy
The backbone of maintenance therapy in most protocols includes daily oral mercaptopurine and weekly oral or parenteral methotrexate. On many protocols, intrathecal chemotherapy for CNS sanctuary therapy is continued during maintenance therapy. Also, vincristine/steroid pulses during maintenance are used by some groups but not others (see below). It is imperative to carefully monitor children on maintenance therapy for both drug-related toxicity and for compliance with the oral chemotherapy agents used during maintenance therapy.[
In the past, clinical practice generally called for the administration of oral mercaptopurine in the evening, on the basis of evidence from older studies that this practice may improve EFS.[
Some patients may develop severe hematologic toxicity when receiving conventional dosages of mercaptopurine because of an inherited deficiency (homozygous mutant) of thiopurine S-methyltransferase, an enzyme that inactivates mercaptopurine.[
Evidence (maintenance therapy):
- A meta-analysis of randomized trials compared thiopurines and found the following:
- Thioguanine did not improve the overall EFS, although particular subgroups may benefit from its use.[
109 ] - The use of continuous thioguanine instead of mercaptopurine during the maintenance phase is associated with an increased risk of hepatic complications, including veno-occlusive disease (sinusoidal obstruction syndrome) and portal hypertension.[
110 ,111 ,112 ,113 ,114 ] - Because of the increased toxicity of thioguanine, mercaptopurine remains the standard drug of choice.
- Thioguanine did not improve the overall EFS, although particular subgroups may benefit from its use.[
- In the COG AALL0932 (NCT01190930) trial, NCI standard-risk patients with average-risk features were randomly assigned to receive weekly oral methotrexate during maintenance at one of two starting doses: 20 mg/m2 (standard) or 40 mg/m2 (investigational).[
115 ][Level of evidence A1]- There was no significant difference in 5-year DFS from the start of maintenance therapy between the two treatment arms (5-year DFS rate, 95.1% for patients who received the standard dose vs. 94.2% for patients who received the investigational dose; P = .92), indicating no advantage for the higher dose of oral methotrexate.
- An intensified maintenance regimen, consisting of rotating pairs of agents, including cyclophosphamide and epipodophyllotoxins along with more standard maintenance agents, has been evaluated in several clinical trials conducted by SJCRH and other groups.[
2 ]- The intensified maintenance with rotating pairs of agents was associated with more episodes of febrile neutropenia [
116 ] and a higher risk of secondary acute myelogenous leukemia,[117 ,118 ] especially when epipodophyllotoxins were included.[116 ]On the basis of these findings, SJCRH modified the agents used in the rotating pair schedule during the maintenance phase. On the Total XV study, standard-risk and high-risk patients received three rotating pairs (mercaptopurine plus methotrexate, cyclophosphamide plus cytarabine, and dexamethasone plus vincristine) throughout this treatment phase. Low-risk patients received more standard maintenance (without cyclophosphamide and cytarabine).[
59 ] - A randomized study from Argentina demonstrated no benefit from this intensified approach compared with a more standard maintenance regimen for patients who receive induction and consolidation phases based on a BFM backbone.[
116 ]
- The intensified maintenance with rotating pairs of agents was associated with more episodes of febrile neutropenia [
Vincristine/corticosteroid pulses
Pulses of vincristine and corticosteroid are often added to the standard maintenance backbone, although the benefit of these pulses within the context of contemporary multiagent chemotherapy regimens remains controversial.
Evidence (vincristine/corticosteroid pulses):
- A CCG randomized trial conducted in the 1980s demonstrated improved outcome in patients who received monthly vincristine/prednisone pulses.[
119 ] - A meta-analysis combining data from six clinical trials from the same treatment era showed an EFS advantage for vincristine/prednisone pulses.[
120 ,121 ] However, overall EFS from these trials was lower than is observed with more contemporary regimens. - A systematic review of the impact of vincristine plus steroid pulses from more recent clinical trials raised the question of whether such pulses are of value in current ALL treatment, which includes more intensive early therapy and risk stratification incorporating early response (MRD) and biological factors.[
121 ] - In a multicenter randomized trial in children with intermediate-risk ALL being treated on a BFM regimen, there was no benefit associated with the addition of six pulses of vincristine/dexamethasone during the continuation phase, although the pulses were administered less frequently than in other trials in which a benefit had been demonstrated.[
122 ] - A small multicenter trial of average-risk patients demonstrated superior EFS in patients receiving vincristine plus corticosteroid pulses. In this study, there was no difference in outcome based on type of steroid (prednisone vs. dexamethasone).[
123 ][Level of evidence A1] - In the COG AALL0932 (NCT01190930) trial, standard-risk patients were randomly assigned during maintenance to receive vincristine/dexamethasone pulses every 4 weeks or every 12 weeks.[
115 ][Level of evidence A1]- For the randomly assigned standard-risk patients, the 5-year DFS rate from the start of maintenance was 94.6%. There was no significant difference between the every-4-week group and the every-12-week group.
- The Chinese Children's Cancer Group conducted a randomized noninferiority trial to determine whether vincristine/dexamethasone pulses could be omitted during the second year of maintenance therapy. One year after the initiation of therapy, 5,054 patients with non-BCR::ABL1 fusion–positive ALL (B-ALL and T-ALL, aged 0–18 years) were randomly assigned to receive either vincristine/dexamethasone pulses every 8 weeks (seven pulses total) or no pulses during the second year of maintenance chemotherapy. Noninferiority was defined by calculating the one-sided 95% upper confidence bound of the difference in EFS probability between arms to ensure that an EFS decrement of 5% or more was ruled out.[
124 ]- For low-risk patients (NCI standard-risk B-ALL with high hyperdiploidy or ETV6::RUNX1 and low end-induction MRD), the EFS difference between arms met the protocol definition of noninferiority, indicating that omission of vincristine/dexamethasone pulses during the second year of maintenance did not result in a decrement of EFS that was greater than 5%.
- For intermediate-risk and high-risk patients, the difference in 5-year EFS between arms did not meet the protocol definition of noninferiority (the 95% upper confidence bound for the difference was 0.055, which exceeded the preset noninferiority margin of 0.05); therefore, it could not be concluded that vincristine/dexamethasone pulses could be omitted in these patients without resulting in an EFS decrement exceeding 5%.
- A systematic review and meta-analysis evaluated the effect of reducing vincristine/steroid pulses on EFS, OS, and toxicity in patients with B-ALL. Twenty-five publications that included more than 12,000 patients were examined.[
125 ]- This study demonstrated that the benefit of these pulses noted in historical trials was not seen in contemporary trials.
- However, there was an increased risk of grade 3+ nonhepatic toxicity in the high-pulse frequency group.
- The authors concluded that decreasing or removing pulses likely does not affect survival or risk of relapse, but it is associated with reduced toxicity.
For regimens that include vincristine/steroid pulses, a number of studies have addressed which steroid (dexamethasone or prednisone) should be used. From these studies, it appears that dexamethasone is associated with superior EFS, but also may lead to a greater frequency of steroid-associated complications, including bone toxicity and infections, especially in older children and adolescents.[
Evidence (dexamethasone vs. prednisone):
- In a CCG study, dexamethasone was compared with prednisone during the induction and maintenance phases for children aged 1 to younger than 10 years with lower-risk ALL.[
6 ,71 ]- Patients randomly assigned to receive dexamethasone had significantly fewer CNS relapses and a significantly better EFS rate.
- In a MRC United Kingdom Acute Lymphoblastic Leukaemia (UKALL) trial, dexamethasone was compared with prednisolone during the induction and maintenance phases in both standard-risk and high-risk patients.[
7 ]- The EFS and incidence of both CNS and non-CNS relapses improved with the use of dexamethasone.
- Dexamethasone was associated with an increased risk of steroid-associated toxicities, including behavioral problems, myopathy, and osteopenia.
- In a DFCI ALL Consortium trial, patients were randomly assigned to receive either dexamethasone or prednisone during all postinduction treatment phases.[
24 ]- Dexamethasone was associated with a superior EFS, but also with a higher frequency of infections (primarily episodes of bacteremia) and, in patients aged 10 years or older, an increased incidence of osteonecrosis and fracture.
The benefit of using dexamethasone in children aged 10 to 18 years requires further investigation because of the increased risk of steroid-induced osteonecrosis in this age group.[
Duration of maintenance therapy
Maintenance chemotherapy generally continues for 2 to 3 years of continuous CR. On some studies, boys are treated longer than girls.[
Adherence to oral medications during maintenance therapy
Nonadherence to treatment with mercaptopurine during maintenance therapy is associated with a significant risk of relapse.[
Evidence (adherence to treatment):
- The COG AALL03N1 (NCT00268528) trial studied the impact of nonadherence to mercaptopurine during maintenance therapy in 327 children and adolescents (169 Hispanic patients and 158 non-Hispanic White patients).[
99 ]- A progressive increase in relapse was observed with decreasing adherence to mercaptopurine, with HRs ranging between 4.0% to 5.7% for adherence rates ranging from 94.9% to 90%, 89.9% to 85%, and less than 85%. After adjusting for other prognostic factors (including NCI risk group and chromosomal abnormalities), a progressive increase in relapse was observed with decreasing adherence to mercaptopurine. MRD data were unavailable in this study population, so they were not included in the analysis of prognostic factors.
- Adherence was significantly lower among Hispanic patients, patients older than 12 years, and patients from single-mother households. However, among adherent patients, Hispanic ethnicity remained an independent predictor of adverse outcome.
- The AALL03N1 trial also included 71 Asian American patients and 68 African American patients, in addition to the non-Hispanic White and Hispanic patients described above.[
100 ]- Using an adherence rate of less than 90% to define nonadherence, 20.5% of the participants were not adherent.
- An adherence rate of less than 90% was associated with increased relapse risk (HR, 3.9).
- Adherence rates were significantly lower in Asian American and African American patients than in non-Hispanic White patients.
- In a third publication from the AALL03N1 study, the following key observations were made:[
129 ]- Patients with mercaptopurine nonadherence (defined as mean adherence rate of <95%) were at a 2.7-fold increased risk of relapse compared with adherers.
- Among adherers, high intra-individual variability in thioguanine levels (due to varying dose-intensity and drug treatment interruptions) was associated with increased risk of relapse.
- The authors of the above studies also found that self-reporting was not a reliable measure of adherence, with 84% of patients overreporting compliance with taking mercaptopurine at least some of the time.[
130 ] The data suggest that additional measures of adherence besides self-reporting are needed. - Another publication from the AALL03N1 study described mercaptopurine ingestion habits, red cell thioguanine nucleotide (TGN) levels, adherence, and relapse risk.[
103 ]- The findings showed that certain ingestion habits (e.g., taking with dairy and taking at varying times throughout the day) were associated with nonadherence. However, after adjusting for adherence and other prognostic factors, ingestion habits were not associated with relapse risk.
- For adherent patients, there was no association between TGN levels and ingestion habits.
- The authors concluded that commonly practiced restrictions surrounding mercaptopurine ingestion do not appear to impact outcome but may hinder adherence.
Treatment options under clinical evaluation
Risk-based treatment assignment is a key therapeutic strategy utilized for children with ALL, and protocols are designed for specific patient populations that have varying degrees of risk of treatment failure. The Risk-Based Treatment Assignment section of this summary describes the clinical and laboratory features used for the initial stratification of children with ALL into risk-based treatment groups.
Information about NCI-supported clinical trials can be found on the
The following are examples of national and/or institutional clinical trials that are currently being conducted:
COG studies for B-ALL
Standard-risk ALL
- COG-AALL1731 (NCT03914625) (A Phase III Trial Investigating Blinatumomab in Combination with Chemotherapy in Patients with Newly Diagnosed Standard Risk or Down Syndrome B-ALL and the Treatment of Patients with Localized B-Lymphoblastic Lymphoma): This protocol is open for NCI standard-risk B-ALL non-Down syndrome patients and all B-ALL patients with Down syndrome (age <31 years) regardless of presenting WBC. The protocol is testing whether the addition of the bispecific T-cell engaging antibody blinatumomab can improve outcome and whether reducing duration of treatment in boys (from 3 years from the start of interim maintenance 1 phase to 2 years from the start of that phase) will adversely impact DFS.
All patients receive a three-drug induction with dexamethasone (no anthracycline). After completion of induction, patients are classified into one of three groups on the basis of biology and early response measures:
- Standard-risk favorable: Presence of either ETV6::RUNX1 or double trisomy (chromosomes 4 and 10), day 8 peripheral blood MRD of <1% and day 29 marrow MRD of <0.01%.
- Standard-risk average: Favorable biology but day 8 peripheral blood MRD of >1% (but day 29 marrow MRD of <0.01%); or presence of double trisomy and day 29 marrow MRD of ≥0.01% but <0.1%; or neutral cytogenetics with day 29 marrow MRD of <0.01%.
- Standard-risk high: Presence of ETV6::RUNX1 or neutral cytogenetics and day 29 marrow MRD of ≥0.01%; or presence of double trisomy and day 29 MRD of ≥0.1%; or presence of neutral cytogenetics and CNS2 at diagnosis, regardless of early response measures; or presence of unfavorable cytogenetics (iAMP21, KMT2A rearrangement, hypodiploidy (<44 chromosomes), or TCF3::HLF (t(17;19)).
Standard-risk favorable patients will be treated with standard therapy.
All standard-risk average patients will have MRD evaluated at day 29 of induction using high-throughput sequencing (HTS)-MRD assay. HTS-MRD undetectable patients will be treated with standard therapy, while patients with HTS-MRD detectable disease (or if HTS-MRD is indeterminate or unavailable), as well as those with double trisomies and day 29 marrow MRD of ≥0.01% to <0.1% will be eligible to participate in a randomization of standard therapy or standard therapy plus the addition of two cycles of blinatumomab.
Standard-risk high patients will be treated with the augmented BFM (NCI high risk) backbone. Any patients with end-consolidation MRD of >1% are removed from protocol therapy. Those with end-consolidation MRD of <0.1% will be eligible to participate in a randomization of either the NCI high-risk backbone alone or this therapy plus two cycles of blinatumomab. Those with end-consolidation MRD of ≥0.1% and <1% will be directly assigned to receive NCI high-risk backbone therapy plus two cycles of blinatumomab.
NCI standard-risk Down syndrome patients who meet definition of standard-risk average will be treated in the same way as non-Down syndrome standard-risk average patients, as detailed above. All other Down syndrome patients, including NCI high-risk Down syndrome patients, those with unfavorable biology, and those with high day 29 MRD will be considered Down syndrome-high, and will be nonrandomly assigned to receive two cycles of blinatumomab added to a deintensified chemotherapy regimen that omits intensive elements of the augmented BFM treatment backbone. Omitted elements include anthracyclines during induction and cyclophosphamide/cytarabine-based chemotherapy during the second half of delayed intensification.
All patients, regardless of risk group, will receive the same duration of therapy (2 years from the start of interim maintenance 1 phase). This represents a reduction in treatment duration by 1 year for boys compared with COG standard treatment.
High-risk and very high-risk ALL
- COG-AALL1732 (NCT03959085) (A Phase III Randomized Trial of Inotuzumab Ozogamicin for Newly Diagnosed High-Risk B-ALL; Risk-Adapted Postinduction Therapy for High-Risk B-ALL, Mixed Phenotype Acute Leukemia [MPAL], and Disseminated B-Lymphoblastic Lymphoma): This protocol is temporarily closed for patients with NCI high-risk non-Down syndrome ALL, any patient with MPAL, and patients with disseminated B-lymphoblastic lymphoma. Patients with NCI standard-risk B-ALL who had steroid pretreatment, CNS3 status, or testicular disease at diagnosis are also eligible for this study.
For patients with B-ALL, the protocol is testing whether the addition of two blocks of inotuzumab ozogamicin to a modified-BFM backbone will improve DFS and whether reducing duration of treatment in boys (from 3 years from the start of interim maintenance 1 phase to 2 years from the start of that phase) will adversely impact DFS. The study also aims to determine the EFS of patients with MPAL and disseminated B-lymphoblastic lymphoma who are treated with a standard high-risk ALL chemotherapy regimen.
All patients receive a four-drug induction (including prednisone and daunorubicin). After completion of induction, subsequent therapy depends on age, biology, and response to therapy.
- High-risk favorable: Patients who are younger than 10 years with ETV6::RUNX1 fusions or high hyperdiploidy with trisomies of chromosomes 4 and 10 and who achieve an MRD of <0.01% at end of induction will receive a modified-BFM regimen with one interim maintenance phase (high-dose methotrexate), and are not eligible for randomization.
- Other high-risk B-ALL patients who do not meet high-risk favorable criteria but who achieve an MRD of <0.01% (for NCI high risk) or <1% (for NCI standard risk) by the end of consolidation (EOC) will be eligible for randomization to modified-BFM therapy with or without two blocks of inotuzumab. Patients who are CD22 negative at diagnosis (or have unknown CD22 status) are not eligible to be randomized, and they are removed from protocol therapy.
- Patients with MPAL and disseminated B-lymphoblastic lymphoma will receive a standard high-risk modified-BFM backbone with two interim maintenance phases, but are not eligible for randomization.
All patients will receive the same duration of therapy (2 years from the start of interim maintenance 1 phase). This represents a reduction in treatment duration by 1 year for boys, compared with standard treatment. NCI high-risk B-ALL patients with EOC MRD of ≥0.01% are removed from protocol therapy and are eligible to enroll on the COG-AALL1721 trial (see above). NCI standard-risk patients with EOC MRD of ≥1% are removed from protocol therapy and are not eligible for enrollment on the COG-AALL1721 trial.
- COG-AALL1721 (NCT03876769) (Study of Efficacy and Safety of Tisagenlecleucel in High-Risk B-ALL End-of-Consolidation MRD-Positive Patients): This protocol is open to patients with NCI high-risk B-ALL who are aged 1 to 25 years, were in morphological CR at end of induction and have end-consolidation MRD of ≥0.01%. The primary objective of the trial is to evaluate the efficacy of tisagenlecleucel (a CD19-directed chimeric antigen receptor [CAR] T cell) as definitive therapy in this patient population, specifically to determine whether the 5-year DFS rate with tisagenlecleucel therapy exceeds 55%.
Patients enrolled on this trial will undergo leukapheresis to collect autologous T cells, which will then be sent for manufacturing of tisagenlecleucel. While awaiting completion of manufacturing, patients will proceed with interim maintenance phase 1 (high-dose methotrexate); this phase may be interrupted as soon as product is available. Once available, patients will then receive lymphodepleting chemotherapy and infusion of tisagenlecleucel. No further anti-leukemic treatment is to be administered after tisagenlecleucel. Marrow samples will be obtained at regular intervals postinfusion, beginning at day 29 after tisagenlecleucel administration to assess disease status; tests of peripheral blood will also be sent to screen for evidence of B-cell aplasia.
Patients must have evidence of CD19-positivity at diagnosis to enroll on trial. Patients with M3 marrow at end of induction, M2/M3 marrow at end of consolidation, hypodiploidy (<44 chromosomes), BCR::ABL1 ALL, or previous treatment with tyrosine kinase inhibitors are excluded from enrollment.
- AALL1631 (NCT03007147) (Imatinib Mesylate and Combination Chemotherapy in Treating Patients With Newly Diagnosed BCR::ABL1 [Ph+] ALL): AALL1631 is an international collaborative protocol conducted by the COG and the European EsPhALL group. Patients with BCR::ABL1-like ALL and ABL-class fusions (defined as those involving ABL1, ABL2, CSF1R, PDGFRB, or PDGFRA) are eligible to enroll. These patients enter the trial after completion of the first month of treatment (induction IA) and receive chemotherapy combined with imatinib (pretreatment using imatinib during induction IA is allowed). After the induction IB phase (weeks 10–12), MRD is assessed by immunoglobulin H/T-cell receptor (IgH-TCR) PCR, and patients are classified as either standard risk (MRD <0.05%) or high risk (MRD >0.05%). Standard-risk patients are randomly assigned to receive one of the following two cytotoxic chemotherapy backbones:
- The EsPhALL backbone used in previous EsPhALL protocols and COG AALL1122; or
- A less-intensive regimen similar to those typically administered to non-BCR::ABL1, high-risk B-ALL patients on COG trials.
Standard-risk patients on both arms will continue to receive imatinib until the completion of all planned chemotherapy (2 years of treatment). The objective of the standard-risk randomization is to determine whether the less-intensive chemotherapy backbone is associated with a similar DFS and lower rates of treatment-related morbidity and mortality compared with the standard therapy (EsPhALL chemotherapy backbone).
High-risk patients will proceed to HSCT after completion of three consolidation blocks of chemotherapy. Treatment with imatinib will restart after HSCT and be administered from day 56 until day 365. The aim is to test the feasibility of post-HSCT administration of imatinib and describe the outcomes of these patients.
Current Clinical Trials
Use our
References:
- Möricke A, Zimmermann M, Reiter A, et al.: Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia 24 (2): 265-84, 2010.
- Pui CH, Pei D, Sandlund JT, et al.: Long-term results of St Jude Total Therapy Studies 11, 12, 13A, 13B, and 14 for childhood acute lymphoblastic leukemia. Leukemia 24 (2): 371-82, 2010.
- Silverman LB, Stevenson KE, O'Brien JE, et al.: Long-term results of Dana-Farber Cancer Institute ALL Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1985-2000). Leukemia 24 (2): 320-34, 2010.
- Oudot C, Auclerc MF, Levy V, et al.: Prognostic factors for leukemic induction failure in children with acute lymphoblastic leukemia and outcome after salvage therapy: the FRALLE 93 study. J Clin Oncol 26 (9): 1496-503, 2008.
- Salzer WL, Devidas M, Carroll WL, et al.: Long-term results of the pediatric oncology group studies for childhood acute lymphoblastic leukemia 1984-2001: a report from the children's oncology group. Leukemia 24 (2): 355-70, 2010.
- Bostrom BC, Sensel MR, Sather HN, et al.: Dexamethasone versus prednisone and daily oral versus weekly intravenous mercaptopurine for patients with standard-risk acute lymphoblastic leukemia: a report from the Children's Cancer Group. Blood 101 (10): 3809-17, 2003.
- Mitchell CD, Richards SM, Kinsey SE, et al.: Benefit of dexamethasone compared with prednisolone for childhood acute lymphoblastic leukaemia: results of the UK Medical Research Council ALL97 randomized trial. Br J Haematol 129 (6): 734-45, 2005.
- Larsen EC, Devidas M, Chen S, et al.: Dexamethasone and High-Dose Methotrexate Improve Outcome for Children and Young Adults With High-Risk B-Acute Lymphoblastic Leukemia: A Report From Children's Oncology Group Study AALL0232. J Clin Oncol 34 (20): 2380-8, 2016.
- Möricke A, Zimmermann M, Valsecchi MG, et al.: Dexamethasone vs prednisone in induction treatment of pediatric ALL: results of the randomized trial AIEOP-BFM ALL 2000. Blood 127 (17): 2101-12, 2016.
- McNeer JL, Nachman JB: The optimal use of steroids in paediatric acute lymphoblastic leukaemia: no easy answers. Br J Haematol 149 (5): 638-52, 2010.
- Silverman LB, Supko JG, Stevenson KE, et al.: Intravenous PEG-asparaginase during remission induction in children and adolescents with newly diagnosed acute lymphoblastic leukemia. Blood 115 (7): 1351-3, 2010.
- Rizzari C, Citterio M, Zucchetti M, et al.: A pharmacological study on pegylated asparaginase used in front-line treatment of children with acute lymphoblastic leukemia. Haematologica 91 (1): 24-31, 2006.
- Place AE, Stevenson KE, Vrooman LM, et al.: Intravenous pegylated asparaginase versus intramuscular native Escherichia coli L-asparaginase in newly diagnosed childhood acute lymphoblastic leukaemia (DFCI 05-001): a randomised, open-label phase 3 trial. Lancet Oncol 16 (16): 1677-90, 2015.
- Asselin BL, Whitin JC, Coppola DJ, et al.: Comparative pharmacokinetic studies of three asparaginase preparations. J Clin Oncol 11 (9): 1780-6, 1993.
- Avramis VI, Sencer S, Periclou AP, et al.: A randomized comparison of native Escherichia coli asparaginase and polyethylene glycol conjugated asparaginase for treatment of children with newly diagnosed standard-risk acute lymphoblastic leukemia: a Children's Cancer Group study. Blood 99 (6): 1986-94, 2002.
- Tram Henriksen L, Gottschalk Højfeldt S, Schmiegelow K, et al.: Prolonged first-line PEG-asparaginase treatment in pediatric acute lymphoblastic leukemia in the NOPHO ALL2008 protocol-Pharmacokinetics and antibody formation. Pediatr Blood Cancer 64 (12): , 2017.
- Schore RJ, Devidas M, Bleyer A, et al.: Plasma asparaginase activity and asparagine depletion in acute lymphoblastic leukemia patients treated with pegaspargase on Children's Oncology Group AALL07P4. Leuk Lymphoma 60 (7): 1740-1748, 2019.
- Jeha S, Pei D, Choi J, et al.: Improved CNS Control of Childhood Acute Lymphoblastic Leukemia Without Cranial Irradiation: St Jude Total Therapy Study 16. J Clin Oncol 37 (35): 3377-3391, 2019.
- Kloos RQH, Pieters R, Jumelet FMV, et al.: Individualized Asparaginase Dosing in Childhood Acute Lymphoblastic Leukemia. J Clin Oncol 38 (7): 715-724, 2020.
- Gupta S, Wang C, Raetz EA, et al.: Impact of Asparaginase Discontinuation on Outcome in Childhood Acute Lymphoblastic Leukemia: A Report From the Children's Oncology Group. J Clin Oncol 38 (17): 1897-1905, 2020.
- van der Sluis IM, Vrooman LM, Pieters R, et al.: Consensus expert recommendations for identification and management of asparaginase hypersensitivity and silent inactivation. Haematologica 101 (3): 279-85, 2016.
- Bleyer A, Asselin BL, Koontz SE, et al.: Clinical application of asparaginase activity levels following treatment with pegaspargase. Pediatr Blood Cancer 62 (6): 1102-5, 2015.
- Tong WH, Pieters R, Kaspers GJ, et al.: A prospective study on drug monitoring of PEGasparaginase and Erwinia asparaginase and asparaginase antibodies in pediatric acute lymphoblastic leukemia. Blood 123 (13): 2026-33, 2014.
- Vrooman LM, Stevenson KE, Supko JG, et al.: Postinduction dexamethasone and individualized dosing of Escherichia Coli L-asparaginase each improve outcome of children and adolescents with newly diagnosed acute lymphoblastic leukemia: results from a randomized study--Dana-Farber Cancer Institute ALL Consortium Protocol 00-01. J Clin Oncol 31 (9): 1202-10, 2013.
- Gottschalk Højfeldt S, Grell K, Abrahamsson J, et al.: Relapse risk following truncation of pegylated asparaginase in childhood acute lymphoblastic leukemia. Blood 137 (17): 2373-2382, 2021.
- van der Sluis IM, Brigitha LJ, Fiocco M, et al.: Continuous PEGasparaginase Dosing Reduces Hypersensitivity Reactions in Pediatric ALL: A Dutch Childhood Oncology Group ALL11 Randomized Trial. J Clin Oncol 42 (14): 1676-1686, 2024.
- Li RJ, Jin R, Liu C, et al.: FDA Approval Summary: Calaspargase Pegol-mknl For Treatment of Acute Lymphoblastic Leukemia in Children and Young Adults. Clin Cancer Res 26 (2): 328-331, 2020.
- Angiolillo AL, Schore RJ, Devidas M, et al.: Pharmacokinetic and pharmacodynamic properties of calaspargase pegol Escherichia coli L-asparaginase in the treatment of patients with acute lymphoblastic leukemia: results from Children's Oncology Group Study AALL07P4. J Clin Oncol 32 (34): 3874-82, 2014.
- Vrooman LM, Blonquist TM, Stevenson KE, et al.: Efficacy and Toxicity of Pegaspargase and Calaspargase Pegol in Childhood Acute Lymphoblastic Leukemia: Results of DFCI 11-001. J Clin Oncol 39 (31): 3496-3505, 2021.
- Salzer WL, Asselin B, Supko JG, et al.: Erwinia asparaginase achieves therapeutic activity after pegaspargase allergy: a report from the Children's Oncology Group. Blood 122 (4): 507-14, 2013.
- Vrooman LM, Kirov II, Dreyer ZE, et al.: Activity and Toxicity of Intravenous Erwinia Asparaginase Following Allergy to E. coli-Derived Asparaginase in Children and Adolescents With Acute Lymphoblastic Leukemia. Pediatr Blood Cancer 63 (2): 228-33, 2016.
- Maese L, Loh ML, Choi MR, et al.: Recombinant Erwinia asparaginase (JZP458) in acute lymphoblastic leukemia: results from the phase 2/3 AALL1931 study. Blood 141 (7): 704-712, 2023.
- Escherich G, Zimmermann M, Janka-Schaub G, et al.: Doxorubicin or daunorubicin given upfront in a therapeutic window are equally effective in children with newly diagnosed acute lymphoblastic leukemia. A randomized comparison in trial CoALL 07-03. Pediatr Blood Cancer 60 (2): 254-7, 2013.
- Pui CH, Sandlund JT, Pei D, et al.: Improved outcome for children with acute lymphoblastic leukemia: results of Total Therapy Study XIIIB at St Jude Children's Research Hospital. Blood 104 (9): 2690-6, 2004.
- Schrappe M, Reiter A, Ludwig WD, et al.: Improved outcome in childhood acute lymphoblastic leukemia despite reduced use of anthracyclines and cranial radiotherapy: results of trial ALL-BFM 90. German-Austrian-Swiss ALL-BFM Study Group. Blood 95 (11): 3310-22, 2000.
- Moghrabi A, Levy DE, Asselin B, et al.: Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95-01 for children with acute lymphoblastic leukemia. Blood 109 (3): 896-904, 2007.
- Prucker C, Attarbaschi A, Peters C, et al.: Induction death and treatment-related mortality in first remission of children with acute lymphoblastic leukemia: a population-based analysis of the Austrian Berlin-Frankfurt-Münster study group. Leukemia 23 (7): 1264-9, 2009.
- Buchmann S, Schrappe M, Baruchel A, et al.: Remission, treatment failure, and relapse in pediatric ALL: an international consensus of the Ponte-di-Legno Consortium. Blood 139 (12): 1785-1793, 2022.
- Balduzzi A, Valsecchi MG, Uderzo C, et al.: Chemotherapy versus allogeneic transplantation for very-high-risk childhood acute lymphoblastic leukaemia in first complete remission: comparison by genetic randomisation in an international prospective study. Lancet 366 (9486): 635-42, 2005 Aug 20-26.
- Silverman LB, Gelber RD, Young ML, et al.: Induction failure in acute lymphoblastic leukemia of childhood. Cancer 85 (6): 1395-404, 1999.
- Schrappe M, Hunger SP, Pui CH, et al.: Outcomes after induction failure in childhood acute lymphoblastic leukemia. N Engl J Med 366 (15): 1371-81, 2012.
- Raetz EA, Rebora P, Conter V, et al.: Outcome for Children and Young Adults With T-Cell ALL and Induction Failure in Contemporary Trials. J Clin Oncol 41 (32): 5025-5034, 2023.
- Dunsmore KP, Winter SS, Devidas M, et al.: Children's Oncology Group AALL0434: A Phase III Randomized Clinical Trial Testing Nelarabine in Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia. J Clin Oncol 38 (28): 3282-3293, 2020.
- Gaynon PS, Desai AA, Bostrom BC, et al.: Early response to therapy and outcome in childhood acute lymphoblastic leukemia: a review. Cancer 80 (9): 1717-26, 1997.
- Borowitz MJ, Devidas M, Hunger SP, et al.: Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children's Oncology Group study. Blood 111 (12): 5477-85, 2008.
- Borowitz MJ, Wood BL, Devidas M, et al.: Prognostic significance of minimal residual disease in high risk B-ALL: a report from Children's Oncology Group study AALL0232. Blood 126 (8): 964-71, 2015.
- van Dongen JJ, Seriu T, Panzer-Grümayer ER, et al.: Prognostic value of minimal residual disease in acute lymphoblastic leukaemia in childhood. Lancet 352 (9142): 1731-8, 1998.
- Zhou J, Goldwasser MA, Li A, et al.: Quantitative analysis of minimal residual disease predicts relapse in children with B-lineage acute lymphoblastic leukemia in DFCI ALL Consortium Protocol 95-01. Blood 110 (5): 1607-11, 2007.
- Conter V, Bartram CR, Valsecchi MG, et al.: Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood 115 (16): 3206-14, 2010.
- Wood B, Wu D, Crossley B, et al.: Measurable residual disease detection by high-throughput sequencing improves risk stratification for pediatric B-ALL. Blood 131 (12): 1350-1359, 2018.
- Vora A, Goulden N, Mitchell C, et al.: Augmented post-remission therapy for a minimal residual disease-defined high-risk subgroup of children and young people with clinical standard-risk and intermediate-risk acute lymphoblastic leukaemia (UKALL 2003): a randomised controlled trial. Lancet Oncol 15 (8): 809-18, 2014.
- Coustan-Smith E, Sancho J, Behm FG, et al.: Prognostic importance of measuring early clearance of leukemic cells by flow cytometry in childhood acute lymphoblastic leukemia. Blood 100 (1): 52-8, 2002.
- Basso G, Veltroni M, Valsecchi MG, et al.: Risk of relapse of childhood acute lymphoblastic leukemia is predicted by flow cytometric measurement of residual disease on day 15 bone marrow. J Clin Oncol 27 (31): 5168-74, 2009.
- Schrappe M, Valsecchi MG, Bartram CR, et al.: Late MRD response determines relapse risk overall and in subsets of childhood T-cell ALL: results of the AIEOP-BFM-ALL 2000 study. Blood 118 (8): 2077-84, 2011.
- Karsa M, Dalla Pozza L, Venn NC, et al.: Improving the identification of high risk precursor B acute lymphoblastic leukemia patients with earlier quantification of minimal residual disease. PLoS One 8 (10): e76455, 2013.
- Nachman JB, Sather HN, Sensel MG, et al.: Augmented post-induction therapy for children with high-risk acute lymphoblastic leukemia and a slow response to initial therapy. N Engl J Med 338 (23): 1663-71, 1998.
- Seibel NL, Steinherz PG, Sather HN, et al.: Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: a report from the Children's Oncology Group. Blood 111 (5): 2548-55, 2008.
- Albertsen BK, Grell K, Abrahamsson J, et al.: Intermittent Versus Continuous PEG-Asparaginase to Reduce Asparaginase-Associated Toxicities: A NOPHO ALL2008 Randomized Study. J Clin Oncol 37 (19): 1638-1646, 2019.
- Pui CH, Campana D, Pei D, et al.: Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med 360 (26): 2730-41, 2009.
- Veerman AJ, Hählen K, Kamps WA, et al.: High cure rate with a moderately intensive treatment regimen in non-high-risk childhood acute lymphoblastic leukemia. Results of protocol ALL VI from the Dutch Childhood Leukemia Study Group. J Clin Oncol 14 (3): 911-8, 1996.
- Chauvenet AR, Martin PL, Devidas M, et al.: Antimetabolite therapy for lesser-risk B-lineage acute lymphoblastic leukemia of childhood: a report from Children's Oncology Group Study P9201. Blood 110 (4): 1105-11, 2007.
- Gustafsson G, Kreuger A, Clausen N, et al.: Intensified treatment of acute childhood lymphoblastic leukaemia has improved prognosis, especially in non-high-risk patients: the Nordic experience of 2648 patients diagnosed between 1981 and 1996. Nordic Society of Paediatric Haematology and Oncology (NOPHO) Acta Paediatr 87 (11): 1151-61, 1998.
- Mattano LA, Devidas M, Maloney KW, et al.: Favorable Trisomies and ETV6-RUNX1 Predict Cure in Low-Risk B-Cell Acute Lymphoblastic Leukemia: Results From Children's Oncology Group Trial AALL0331. J Clin Oncol 39 (14): 1540-1552, 2021.
- Mahoney DH, Shuster JJ, Nitschke R, et al.: Intensification with intermediate-dose intravenous methotrexate is effective therapy for children with lower-risk B-precursor acute lymphoblastic leukemia: A Pediatric Oncology Group study. J Clin Oncol 18 (6): 1285-94, 2000.
- Veerman AJ, Kamps WA, van den Berg H, et al.: Dexamethasone-based therapy for childhood acute lymphoblastic leukaemia: results of the prospective Dutch Childhood Oncology Group (DCOG) protocol ALL-9 (1997-2004). Lancet Oncol 10 (10): 957-66, 2009.
- Schore RJ, Angiolillo AJ, Kairalla JA, et al.: Outcomes with reduced intensity therapy in a low-risk subset of children with National Cancer Institute (NCI) standard-risk (SR) B-lymphoblastic leukemia (B-ALL): A report from Children's Oncology Group (COG) AALL0932. [Abstract] J Clin Oncol 38 (15 suppl): A-10509, 2020.
Also available online . Last accessed June 13, 2022. - Silverman LB, Gelber RD, Dalton VK, et al.: Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood 97 (5): 1211-8, 2001.
- Pession A, Valsecchi MG, Masera G, et al.: Long-term results of a randomized trial on extended use of high dose L-asparaginase for standard risk childhood acute lymphoblastic leukemia. J Clin Oncol 23 (28): 7161-7, 2005.
- Coustan-Smith E, Sancho J, Hancock ML, et al.: Use of peripheral blood instead of bone marrow to monitor residual disease in children with acute lymphoblastic leukemia. Blood 100 (7): 2399-402, 2002.
- Stow P, Key L, Chen X, et al.: Clinical significance of low levels of minimal residual disease at the end of remission induction therapy in childhood acute lymphoblastic leukemia. Blood 115 (23): 4657-63, 2010.
- Gaynon PS, Angiolillo AL, Carroll WL, et al.: Long-term results of the children's cancer group studies for childhood acute lymphoblastic leukemia 1983-2002: a Children's Oncology Group Report. Leukemia 24 (2): 285-97, 2010.
- Riehm H, Gadner H, Henze G, et al.: Results and significance of six randomized trials in four consecutive ALL-BFM studies. Hamatol Bluttransfus 33: 439-50, 1990.
- Hutchinson RJ, Gaynon PS, Sather H, et al.: Intensification of therapy for children with lower-risk acute lymphoblastic leukemia: long-term follow-up of patients treated on Children's Cancer Group Trial 1881. J Clin Oncol 21 (9): 1790-7, 2003.
- Möricke A, Reiter A, Zimmermann M, et al.: Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood 111 (9): 4477-89, 2008.
- Matloub Y, Bostrom BC, Hunger SP, et al.: Escalating intravenous methotrexate improves event-free survival in children with standard-risk acute lymphoblastic leukemia: a report from the Children's Oncology Group. Blood 118 (2): 243-51, 2011.
- Matloub Y, Stork L, Asselin B, et al.: Outcome of Children with Standard-Risk T-Lineage Acute Lymphoblastic Leukemia--Comparison among Different Treatment Strategies. Pediatr Blood Cancer 63 (2): 255-61, 2016.
- Maloney KW, Devidas M, Wang C, et al.: Outcome in Children With Standard-Risk B-Cell Acute Lymphoblastic Leukemia: Results of Children's Oncology Group Trial AALL0331. J Clin Oncol 38 (6): 602-612, 2020.
- Vora A, Goulden N, Wade R, et al.: Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): a randomised controlled trial. Lancet Oncol 14 (3): 199-209, 2013.
- Schrappe M, Bleckmann K, Zimmermann M, et al.: Reduced-Intensity Delayed Intensification in Standard-Risk Pediatric Acute Lymphoblastic Leukemia Defined by Undetectable Minimal Residual Disease: Results of an International Randomized Trial (AIEOP-BFM ALL 2000). J Clin Oncol 36 (3): 244-253, 2018.
- Ariffin H, Chiew EKH, Oh BLZ, et al.: Anthracycline-Free Protocol for Favorable-Risk Childhood ALL: A Noninferiority Comparison Between Malaysia-Singapore ALL 2003 and ALL 2010 Studies. J Clin Oncol 41 (20): 3642-3651, 2023.
- Pui CH, Mahmoud HH, Rivera GK, et al.: Early intensification of intrathecal chemotherapy virtually eliminates central nervous system relapse in children with acute lymphoblastic leukemia. Blood 92 (2): 411-5, 1998.
- Mattano LA, Sather HN, Trigg ME, et al.: Osteonecrosis as a complication of treating acute lymphoblastic leukemia in children: a report from the Children's Cancer Group. J Clin Oncol 18 (18): 3262-72, 2000.
- Aricò M, Valsecchi MG, Conter V, et al.: Improved outcome in high-risk childhood acute lymphoblastic leukemia defined by prednisone-poor response treated with double Berlin-Frankfurt-Muenster protocol II. Blood 100 (2): 420-6, 2002.
- Steinherz PG, Seibel NL, Sather H, et al.: Treatment of higher risk acute lymphoblastic leukemia in young people (CCG-1961), long-term follow-up: a report from the Children's Oncology Group. Leukemia 33 (9): 2144-2154, 2019.
- Mattano LA, Devidas M, Nachman JB, et al.: Effect of alternate-week versus continuous dexamethasone scheduling on the risk of osteonecrosis in paediatric patients with acute lymphoblastic leukaemia: results from the CCG-1961 randomised cohort trial. Lancet Oncol 13 (9): 906-15, 2012.
- Moorman AV, Antony G, Wade R, et al.: Time to Cure for Childhood and Young Adult Acute Lymphoblastic Leukemia Is Independent of Early Risk Factors: Long-Term Follow-Up of the UKALL2003 Trial. J Clin Oncol 40 (36): 4228-4239, 2022.
- Lipshultz SE, Scully RE, Lipsitz SR, et al.: Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: long-term follow-up of a prospective, randomised, multicentre trial. Lancet Oncol 11 (10): 950-61, 2010.
- Barry EV, Vrooman LM, Dahlberg SE, et al.: Absence of secondary malignant neoplasms in children with high-risk acute lymphoblastic leukemia treated with dexrazoxane. J Clin Oncol 26 (7): 1106-11, 2008.
- Asselin BL, Devidas M, Chen L, et al.: Cardioprotection and Safety of Dexrazoxane in Patients Treated for Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia or Advanced-Stage Lymphoblastic Non-Hodgkin Lymphoma: A Report of the Children's Oncology Group Randomized Trial Pediatric Oncology Group 9404. J Clin Oncol 34 (8): 854-62, 2016.
- Schultz KR, Pullen DJ, Sather HN, et al.: Risk- and response-based classification of childhood B-precursor acute lymphoblastic leukemia: a combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children's Cancer Group (CCG). Blood 109 (3): 926-35, 2007.
- van Binsbergen AL, de Haas V, van der Velden VHJ, et al.: Efficacy and toxicity of high-risk therapy of the Dutch Childhood Oncology Group in childhood acute lymphoblastic leukemia. Pediatr Blood Cancer 69 (2): e29387, 2022.
- Schrauder A, Reiter A, Gadner H, et al.: Superiority of allogeneic hematopoietic stem-cell transplantation compared with chemotherapy alone in high-risk childhood T-cell acute lymphoblastic leukemia: results from ALL-BFM 90 and 95. J Clin Oncol 24 (36): 5742-9, 2006.
- Ribera JM, Ortega JJ, Oriol A, et al.: Comparison of intensive chemotherapy, allogeneic, or autologous stem-cell transplantation as postremission treatment for children with very high risk acute lymphoblastic leukemia: PETHEMA ALL-93 Trial. J Clin Oncol 25 (1): 16-24, 2007.
- Pieters R, de Groot-Kruseman H, Van der Velden V, et al.: Successful Therapy Reduction and Intensification for Childhood Acute Lymphoblastic Leukemia Based on Minimal Residual Disease Monitoring: Study ALL10 From the Dutch Childhood Oncology Group. J Clin Oncol 34 (22): 2591-601, 2016.
- Conter V, Valsecchi MG, Parasole R, et al.: Childhood high-risk acute lymphoblastic leukemia in first remission: results after chemotherapy or transplant from the AIEOP ALL 2000 study. Blood 123 (10): 1470-8, 2014.
- Ifversen M, Turkiewicz D, Marquart HV, et al.: Low burden of minimal residual disease prior to transplantation in children with very high risk acute lymphoblastic leukaemia: The NOPHO ALL2008 experience. Br J Haematol 184 (6): 982-993, 2019.
- Pui CH, Rebora P, Schrappe M, et al.: Outcome of Children With Hypodiploid Acute Lymphoblastic Leukemia: A Retrospective Multinational Study. J Clin Oncol 37 (10): 770-779, 2019.
- McNeer JL, Devidas M, Dai Y, et al.: Hematopoietic Stem-Cell Transplantation Does Not Improve the Poor Outcome of Children With Hypodiploid Acute Lymphoblastic Leukemia: A Report From Children's Oncology Group. J Clin Oncol 37 (10): 780-789, 2019.
- Bhatia S, Landier W, Shangguan M, et al.: Nonadherence to oral mercaptopurine and risk of relapse in Hispanic and non-Hispanic white children with acute lymphoblastic leukemia: a report from the children's oncology group. J Clin Oncol 30 (17): 2094-101, 2012.
- Bhatia S, Landier W, Hageman L, et al.: 6MP adherence in a multiracial cohort of children with acute lymphoblastic leukemia: a Children's Oncology Group study. Blood 124 (15): 2345-53, 2014.
- Schmiegelow K, Glomstein A, Kristinsson J, et al.: Impact of morning versus evening schedule for oral methotrexate and 6-mercaptopurine on relapse risk for children with acute lymphoblastic leukemia. Nordic Society for Pediatric Hematology and Oncology (NOPHO). J Pediatr Hematol Oncol 19 (2): 102-9, 1997 Mar-Apr.
- Clemmensen KK, Christensen RH, Shabaneh DN, et al.: The circadian schedule for childhood acute lymphoblastic leukemia maintenance therapy does not influence event-free survival in the NOPHO ALL92 protocol. Pediatr Blood Cancer 61 (4): 653-8, 2014.
- Landier W, Hageman L, Chen Y, et al.: Mercaptopurine Ingestion Habits, Red Cell Thioguanine Nucleotide Levels, and Relapse Risk in Children With Acute Lymphoblastic Leukemia: A Report From the Children's Oncology Group Study AALL03N1. J Clin Oncol 35 (15): 1730-1736, 2017.
- Relling MV, Hancock ML, Rivera GK, et al.: Mercaptopurine therapy intolerance and heterozygosity at the thiopurine S-methyltransferase gene locus. J Natl Cancer Inst 91 (23): 2001-8, 1999.
- Andersen JB, Szumlanski C, Weinshilboum RM, et al.: Pharmacokinetics, dose adjustments, and 6-mercaptopurine/methotrexate drug interactions in two patients with thiopurine methyltransferase deficiency. Acta Paediatr 87 (1): 108-11, 1998.
- Yang JJ, Landier W, Yang W, et al.: Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. J Clin Oncol 33 (11): 1235-42, 2015.
- Moriyama T, Nishii R, Perez-Andreu V, et al.: NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat Genet 48 (4): 367-73, 2016.
- Zhou H, Li L, Yang P, et al.: Optimal predictor for 6-mercaptopurine intolerance in Chinese children with acute lymphoblastic leukemia: NUDT15, TPMT, or ITPA genetic variants? BMC Cancer 18 (1): 516, 2018.
- Escherich G, Richards S, Stork LC, et al.: Meta-analysis of randomised trials comparing thiopurines in childhood acute lymphoblastic leukaemia. Leukemia 25 (6): 953-9, 2011.
- Broxson EH, Dole M, Wong R, et al.: Portal hypertension develops in a subset of children with standard risk acute lymphoblastic leukemia treated with oral 6-thioguanine during maintenance therapy. Pediatr Blood Cancer 44 (3): 226-31, 2005.
- De Bruyne R, Portmann B, Samyn M, et al.: Chronic liver disease related to 6-thioguanine in children with acute lymphoblastic leukaemia. J Hepatol 44 (2): 407-10, 2006.
- Vora A, Mitchell CD, Lennard L, et al.: Toxicity and efficacy of 6-thioguanine versus 6-mercaptopurine in childhood lymphoblastic leukaemia: a randomised trial. Lancet 368 (9544): 1339-48, 2006.
- Jacobs SS, Stork LC, Bostrom BC, et al.: Substitution of oral and intravenous thioguanine for mercaptopurine in a treatment regimen for children with standard risk acute lymphoblastic leukemia: a collaborative Children's Oncology Group/National Cancer Institute pilot trial (CCG-1942). Pediatr Blood Cancer 49 (3): 250-5, 2007.
- Stork LC, Matloub Y, Broxson E, et al.: Oral 6-mercaptopurine versus oral 6-thioguanine and veno-occlusive disease in children with standard-risk acute lymphoblastic leukemia: report of the Children's Oncology Group CCG-1952 clinical trial. Blood 115 (14): 2740-8, 2010.
- Angiolillo AL, Schore RJ, Kairalla JA, et al.: Excellent Outcomes With Reduced Frequency of Vincristine and Dexamethasone Pulses in Standard-Risk B-Lymphoblastic Leukemia: Results From Children's Oncology Group AALL0932. J Clin Oncol 39 (13): 1437-1447, 2021.
- Felice MS, Rossi JG, Gallego MS, et al.: No advantage of a rotational continuation phase in acute lymphoblastic leukemia in childhood treated with a BFM back-bone therapy. Pediatr Blood Cancer 57 (1): 47-55, 2011.
- Hijiya N, Hudson MM, Lensing S, et al.: Cumulative incidence of secondary neoplasms as a first event after childhood acute lymphoblastic leukemia. JAMA 297 (11): 1207-15, 2007.
- Pui CH, Ribeiro RC, Hancock ML, et al.: Acute myeloid leukemia in children treated with epipodophyllotoxins for acute lymphoblastic leukemia. N Engl J Med 325 (24): 1682-7, 1991.
- Bleyer WA, Sather HN, Nickerson HJ, et al.: Monthly pulses of vincristine and prednisone prevent bone marrow and testicular relapse in low-risk childhood acute lymphoblastic leukemia: a report of the CCG-161 study by the Childrens Cancer Study Group. J Clin Oncol 9 (6): 1012-21, 1991.
- Duration and intensity of maintenance chemotherapy in acute lymphoblastic leukaemia: overview of 42 trials involving 12 000 randomised children. Childhood ALL Collaborative Group. Lancet 347 (9018): 1783-8, 1996.
- Eden TO, Pieters R, Richards S, et al.: Systematic review of the addition of vincristine plus steroid pulses in maintenance treatment for childhood acute lymphoblastic leukaemia - an individual patient data meta-analysis involving 5,659 children. Br J Haematol 149 (5): 722-33, 2010.
- Conter V, Valsecchi MG, Silvestri D, et al.: Pulses of vincristine and dexamethasone in addition to intensive chemotherapy for children with intermediate-risk acute lymphoblastic leukaemia: a multicentre randomised trial. Lancet 369 (9556): 123-31, 2007.
- De Moerloose B, Suciu S, Bertrand Y, et al.: Improved outcome with pulses of vincristine and corticosteroids in continuation therapy of children with average risk acute lymphoblastic leukemia (ALL) and lymphoblastic non-Hodgkin lymphoma (NHL): report of the EORTC randomized phase 3 trial 58951. Blood 116 (1): 36-44, 2010.
- Yang W, Cai J, Shen S, et al.: Pulse therapy with vincristine and dexamethasone for childhood acute lymphoblastic leukaemia (CCCG-ALL-2015): an open-label, multicentre, randomised, phase 3, non-inferiority trial. Lancet Oncol 22 (9): 1322-1332, 2021.
- Guolla L, Breitbart S, Foroutan F, et al.: Impact of vincristine-steroid pulses during maintenance for B-cell pediatric ALL: a systematic review and meta-analysis. Blood 141 (24): 2944-2954, 2023.
- Strauss AJ, Su JT, Dalton VM, et al.: Bony morbidity in children treated for acute lymphoblastic leukemia. J Clin Oncol 19 (12): 3066-72, 2001.
- Warris LT, van den Heuvel-Eibrink MM, Aarsen FK, et al.: Hydrocortisone as an Intervention for Dexamethasone-Induced Adverse Effects in Pediatric Patients With Acute Lymphoblastic Leukemia: Results of a Double-Blind, Randomized Controlled Trial. J Clin Oncol 34 (19): 2287-93, 2016.
- Hoppmann AL, Chen Y, Landier W, et al.: Individual prediction of nonadherence to oral mercaptopurine in children with acute lymphoblastic leukemia: Results from COG AALL03N1. Cancer 127 (20): 3832-3839, 2021.
- Bhatia S, Landier W, Hageman L, et al.: Systemic Exposure to Thiopurines and Risk of Relapse in Children With Acute Lymphoblastic Leukemia: A Children's Oncology Group Study. JAMA Oncol 1 (3): 287-95, 2015.
- Landier W, Chen Y, Hageman L, et al.: Comparison of self-report and electronic monitoring of 6MP intake in childhood ALL: a Children's Oncology Group study. Blood 129 (14): 1919-1926, 2017.
Central Nervous System (CNS)-Directed Therapy for Childhood ALL
Overview of CNS-Directed Treatment Regimens
At diagnosis, approximately 3% of patients have CNS3 disease (defined as cerebrospinal fluid [CSF] specimen with ≥5 white blood cells [WBC]/μL with lymphoblasts and/or the presence of cranial nerve palsies). However, unless specific therapy is directed toward the CNS, most children will eventually develop overt CNS leukemia whether or not lymphoblasts were detected in the spinal fluid at initial diagnosis. Therefore, all children with acute lymphoblastic leukemia (ALL) should receive systemic combination chemotherapy together with some form of CNS prophylaxis.
Because the CNS is a sanctuary site (i.e., an anatomical space that is poorly penetrated by many of the systemically administered chemotherapy agents typically used to treat ALL), specific CNS-directed therapies must be instituted early in treatment to eliminate clinically evident CNS disease at diagnosis and to prevent CNS relapse in all patients. Historically, survival rates for children with ALL improved dramatically after CNS-directed therapies were added to treatment regimens.
Standard treatment options for CNS-directed therapy include the following:
- Intrathecal chemotherapy.
- CNS-directed systemic chemotherapy.
- Cranial radiation therapy.
All of these treatment modalities have a role in the treatment and prevention of CNS leukemia. The combination of intrathecal chemotherapy plus CNS-directed systemic chemotherapy is standard. Cranial radiation is reserved for select situations.[
The type of CNS-therapy that is used is based on a patient's risk of CNS-relapse, with higher-risk patients receiving more intensive treatments. Data suggest that the following groups of patients are at increased risk of CNS relapse:
- Patients with 5 or more WBC/µL and blasts in the cerebrospinal fluid (CSF) (CNS3), obtained at diagnosis.
- Patients with blasts in the CSF but fewer than 5 WBC/µL (CNS2) may be at increased risk of CNS relapse,[
2 ] although this risk appears to be nearly fully abrogated if they receive more doses of intrathecal chemotherapy, especially during the induction phase.[3 ] - Patients with T-ALL, especially those with high presenting peripheral blood leukocyte counts.
- Patients who have a traumatic lumbar puncture showing blasts at the time of diagnosis may have an increased risk of CNS relapse. These patients receive more intensive CNS-directed therapy on some treatment protocols.[
3 ,4 ]
CNS-directed treatment regimens for newly diagnosed childhood ALL are presented in Table 11.
| Disease Status | Standard Treatment Options | |
|---|---|---|
| ALL = acute lymphoblastic leukemia; CNS = central nervous system; CNS3 = cerebrospinal fluid with ≥5 white blood cells/µL, cytospin positive for blasts, or cranial nerve palsies. | ||
| a The drug itself is not CNS-penetrant, but leads to cerebrospinal fluid asparagine depletion. | ||
| Standard-risk ALL | Intrathecal chemotherapy | |
| Methotrexate alone | ||
| Methotrexate with cytarabine and hydrocortisone | ||
| CNS-directed systemic chemotherapy | ||
| Dexamethasone | ||
| L-asparaginasea | ||
| High-dose methotrexate with leucovorin rescue | ||
| Escalating-dose intravenous methotrexate (no leucovorin rescue) | ||
| High-risk and very high-risk ALL | Intrathecal chemotherapy | |
| Methotrexate alone | ||
| Methotrexate with cytarabine and hydrocortisone | ||
| CNS-directed systemic chemotherapy | ||
| Dexamethasone | ||
| L-asparaginasea | ||
| High-dose methotrexate with leucovorin rescue | ||
| Cranial radiation therapy | ||
A major goal of current ALL clinical trials is to provide effective CNS therapy while minimizing neurological toxic effects and other late effects.
Intrathecal Chemotherapy
All therapeutic regimens for childhood ALL include intrathecal chemotherapy. Intrathecal chemotherapy is usually started at the beginning of induction, intensified during consolidation and, in many protocols, continued throughout the maintenance phase.
Intrathecal chemotherapy typically consists of one of the following:[
- Methotrexate alone.
- Methotrexate with cytarabine and hydrocortisone (triple intrathecal chemotherapy).
Unlike intrathecal cytarabine, intrathecal methotrexate has a significant systemic effect, which may contribute to prevention of marrow relapse.[
CNS-Directed Systemic Chemotherapy
In addition to therapy delivered directly to the brain and spinal fluid, systemically administered agents are also an important component of effective CNS prophylaxis. The following systemically administered drugs provide some degree of CNS prophylaxis:
- Dexamethasone.
- L-asparaginase (does not penetrate into CSF itself, but leads to CSF asparagine depletion).[
7 ] - High-dose methotrexate with leucovorin rescue.
- Escalating dose intravenous (IV) methotrexate without leucovorin rescue.
Evidence (CNS-directed systemic chemotherapy):
- In a randomized Children's Cancer Group (CCG) study of standard-risk patients who all received the same dose and schedule of intrathecal methotrexate without cranial irradiation, oral dexamethasone was associated with a 50% decrease in the rate of CNS relapse compared with oral prednisone.[
8 ] - In another standard-risk ALL trial (COG-1991), escalating dose IV methotrexate without leucovorin rescue significantly reduced the CNS relapse rate compared with standard, low-dose, oral methotrexate given during each of two interim maintenance phases.[
9 ] - In a randomized clinical trial conducted by the former Pediatric Oncology Group, T-ALL patients who received high-dose methotrexate experienced a significantly lower CNS relapse rate than did patients who did not receive high-dose methotrexate.[
10 ]
Cranial Radiation Therapy
The proportion of patients receiving cranial radiation therapy has decreased significantly over time. At present, most newly diagnosed children with ALL are treated without cranial radiation therapy. Many groups administer cranial radiation therapy only to those patients considered to be at highest risk of subsequent CNS relapse, such as those with documented CNS leukemia at diagnosis (as defined above) (≥5 WBC/μL with blasts; CNS3) and/or T-cell phenotype with high presenting WBC count.[
In patients who do receive radiation therapy, the cranial radiation dose has been significantly reduced and administration of spinal irradiation is not standard.
Ongoing trials seek to determine whether radiation therapy can be eliminated from the treatment of all children with newly diagnosed ALL without compromising survival or leading to increased rate of toxicities from upfront and salvage therapies.[
CNS Therapy for Standard-Risk Patients
Intrathecal chemotherapy without cranial radiation therapy, given in the context of appropriate systemic chemotherapy, results in CNS relapse rates of less than 5% for children with standard-risk ALL.[
The use of cranial radiation therapy is not a necessary component of CNS-directed therapy for these patients.[
Evidence (triple intrathecal chemotherapy vs. intrathecal methotrexate):
- The CCG-1952 study for National Cancer Institute (NCI) standard-risk patients compared the relative efficacy and toxicity of triple intrathecal chemotherapy (methotrexate, cytarabine, and hydrocortisone) with methotrexate as the sole intrathecal agent in nonirradiated patients.[
21 ]- There was no significant difference in either CNS or non-CNS toxicities.
- Although triple intrathecal chemotherapy was associated with a lower rate of isolated CNS relapse (3.4% ± 1.0% compared with 5.9% ± 1.2% for intrathecal methotrexate; P = .004), there was no difference in event-free survival (EFS) rates.
- The reduction in CNS relapse rate was especially notable in patients with CNS2 status at diagnosis (lymphoblasts seen in CSF cytospin, but with <5 WBC/high-power field on CSF cell count). The isolated CNS relapse rate was 7.7% (± 5.3%) for CNS2 patients who received triple intrathecal chemotherapy, compared with 23.0% (± 9.5%) for those who received intrathecal methotrexate alone (P = .04).
- There were more bone marrow relapses in the group that received the triple intrathecal chemotherapy, leading to a worse overall survival (OS) rate (90.3% ± 1.5%) compared with the intrathecal methotrexate group (94.4% ± 1.1%; P = .01).
- When the analysis was restricted to patients with B-ALL and rapid early response (M1 marrow on day 14), there was no difference between triple and single intrathecal chemotherapy in terms of rates of CNS relapse, OS, or EFS rates.
- The findings of this trial need to be interpreted within the context of other therapy administered to patients. Dexamethasone, which has been associated with lower CNS relapse rates and improved EFS rates in standard-risk patients in other trials,[
8 ,22 ] was not used in CCG-1952 (prednisone was the only steroid administered to patients).[23 ] It is not clear whether the results of the CCG-1952 trial are generalizable to protocols that include the use of dexamethasone and/or other CNS-directed systemic therapies.
- In a follow-up study of neurocognitive functioning in the two groups, there were no clinically significant differences.[
24 ][Level of evidence A3]
CNS Therapy for High-Risk and Very High-Risk Patients Without CNS Involvement
Intrathecal chemotherapy
Approaches to intrathecal therapy have also been studied in high-risk patients.
Evidence (triple intrathecal chemotherapy vs. intrathecal methotrexate):
- The COG AALL1131 (NCT02883049) study for NCI high-risk patients and NCI standard-risk B-ALL patients with slow early response (defined by day 8 peripheral blood MRD and/or day 29 marrow MRD) randomly assigned patients aged 1 to 30 years to receive either postinduction intrathecal methotrexate or triple intrathecal chemotherapy (methotrexate, cytarabine, and hydrocortisone). Patients with CNS3 disease were not eligible, and patients on this trial did not receive cranial radiation. Postinduction intrathecal therapy was administered for a total of 21 to 26 doses. Neurocognitive assessments were performed in a subset of patients aged 6 to 12 years at initial diagnosis.[
25 ]- The 5-year postinduction disease-free survival (DFS) rates were 93.2% (± 2.1%) for patients randomly assigned to intrathecal methotrexate and 90.6% (± 2.3%) (P = .85) for patients assigned to triple intrathecal chemotherapy.
- The OS rates were 96.3% (± 1.5%) for patients who received intrathecal methotrexate and 96.7% (± 1.4%) (P = .77) for patients who received triple intrathecal chemotherapy.
- There were no differences in the cumulative incidence of isolated bone marrow relapse, isolated CNS relapse, or combined bone marrow and CNS relapse between the two arms.
- There were no significant differences in neurological toxicities or in assessments of neurocognitive functioning during treatment for patients who received intrathecal methotrexate compared with patients who received triple intrathecal chemotherapy.
Cranial radiation therapy
Controversy exists as to whether high-risk and very high-risk patients should be treated with cranial radiation therapy, although there is a growing consensus that cranial radiation therapy may not be necessary for most of these patients.[
- Patients with T-cell phenotype and high initial WBC count.
- Patients with high-risk B-ALL and extremely high presenting leukocyte counts and/or adverse cytogenetic abnormalities.
Both the proportion of patients receiving radiation therapy and the dose of radiation administered have decreased over the last two decades.
Evidence (cranial radiation therapy):
- In a trial conducted between 1990 and 1995, the Berlin-Frankfurt-Münster (BFM) group demonstrated that a reduced dose of prophylactic radiation (12 Gy instead of 18 Gy) provided effective CNS prophylaxis in high-risk patients.[
26 ] - In the follow-up trial conducted by the BFM group between 1995 and 2000 (BFM-95), cranial radiation therapy was administered to approximately 20% of patients (compared with 70% on the previous trial), including patients with T-cell phenotype, a slow early response (as measured by peripheral blood blast count after a 1-week steroid prophase), and/or adverse cytogenetic abnormalities.[
18 ]- While the rate of isolated CNS relapses was higher in the nonirradiated higher-risk patients compared with historical (irradiated) cohorts, their overall EFS rates were not significantly different.
- Several groups, including the St. Jude Children's Research Hospital (SJCRH), the Dutch Childhood Oncology Group (DCOG), and the European Organization for Research and Treatment of Cancer (EORTC), have published results of trials that omitted cranial radiation therapy for all patients, including high-risk subsets.[
12 ,13 ,27 ] Most of these trials have included at least four doses of high-dose methotrexate during postinduction consolidation and an increased frequency of intrathecal chemotherapy. The SJCRH and DCOG studies also included frequent vincristine/dexamethasone pulses and intensified dosing of pegaspargase,[12 ,13 ] while the EORTC trials included additional high-dose methotrexate and multiple doses of high-dose cytarabine during postinduction treatment phases for patients with CNS3 status (CSF with ≥5 WBC/µL and cytospin positive for blasts).[27 ]- The 5-year cumulative incidence of isolated CNS relapse on those trials was between 2% and 4%, although some patient subsets had a significantly higher rate of CNS relapse. On the SJCRH study, clinical features associated with a significantly higher risk of isolated CNS relapse included T-cell phenotype, the t(1;19) translocation, and the presence of blasts in the CSF at diagnosis.[
12 ] - The overall EFS rate was 85.6% for the SJCRH study and 81% for the DCOG study—both in line with outcomes achieved by contemporaneously conducted clinical trials on which some patients received prophylactic radiation therapy—but was lower on the EORTC trial (8-year EFS rate, 69.6%).[
27 ] - On the SJCRH study, 33 of 498 patients (6.6%) in first remission with high-risk features (including 26 patients with high minimal residual disease [MRD], 6 with BCR::ABL1-positive ALL, and 1 with near haploidy) received an allogeneic hematopoietic stem cell transplant, which included total-body irradiation.[
12 ]
- The 5-year cumulative incidence of isolated CNS relapse on those trials was between 2% and 4%, although some patient subsets had a significantly higher rate of CNS relapse. On the SJCRH study, clinical features associated with a significantly higher risk of isolated CNS relapse included T-cell phenotype, the t(1;19) translocation, and the presence of blasts in the CSF at diagnosis.[
- In a meta-analysis of aggregated data from more than 16,000 patients treated between 1996 and 2007 by ten cooperative groups, the use of cranial radiation therapy did not appear to impact 5-year OS or cumulative incidence of any event.[
14 ]- In subgroup analyses of high-risk subsets, only those with CNS3 status at diagnosis appeared to benefit from cranial radiation, with a significantly lower rate of CNS relapses (isolated/any) in irradiated patients. However, even within this subgroup, OS rates were similar with or without the use of radiation therapy.
- This study suggests that cranial radiation therapy may not be an essential component of treatment, even for high-risk patients. However, interpretation is limited by the considerable variation in treatment administered to patients by the different cooperative groups.
- The EORTC-58832 trial that was conducted between 1983 and 1989 included patients with medium-risk and high-risk ALL. Patients were randomly assigned to receive or not to receive cranial radiation after intensification and before maintenance therapy.[
28 ][Level of evidence A1]- The 25-year EFS and OS rates in the two arms of the trial were similar: the EFS rate was 59.5% and the OS rate was 78.1% for patients who did not receive cranial radiation; the EFS rate was 60.5% and the OS rate was 78.5% for patients who received cranial radiation.
- There was a dramatic decrease in the rate of late CNS adverse events in the patients who did not receive cranial radiation therapy.
- The incidence of second neoplasms was also decreased in patients who did not receive radiation (7.3%) compared with patients who did receive radiation (13%) (HR, 0.43). Meningiomas accounted for the increased incidence of second neoplasms in the cranial radiation group.
CNS Therapy for Patients With CNS3 Disease at Diagnosis
Therapy for ALL patients with clinically evident CNS disease (≥5 WBC/high-power field with blasts on cytospin; cranial nerve palsies-CNS3) at diagnosis typically includes intrathecal chemotherapy and cranial radiation therapy (usual dose is 18 Gy).[
Evidence (cranial radiation therapy):
- The SJCRH, DCOG, and the EORTC have published results of trials that omitted cranial radiation therapy for all patients, including high-risk subsets.[
12 ,27 ] These trials have included at least four doses of high-dose methotrexate during postinduction consolidation and an increased frequency of intrathecal chemotherapy. The SJCRH study also included higher cumulative doses of anthracycline than on Children's Oncology Group (COG) trials, and frequent vincristine/dexamethasone pulses and intensified dosing of pegaspargase,[12 ] while the EORTC trials included additional high-dose methotrexate and multiple doses of high-dose cytarabine, during postinduction treatment phases for CNS3 (CSF with ≥5 WBC/µL and cytospin positive for blasts) patients.[27 ]- On the SJCRH Total XV (TOTXV) study, patients with CNS3 status (N = 9) were treated without cranial radiation therapy (observed 5-year EFS rate, 43% ± 23%; OS rate, 71% ± 22%).[
12 ] On this study, CNS leukemia at diagnosis (defined as CNS3 status or traumatic lumbar puncture with blasts) was an independent predictor of inferior EFS. - On the DCOG-9 trial, the 5-year EFS rate of CNS3 patients (n = 21) treated without cranial radiation therapy was 67% (± 10%).[
13 ] - On the EORTC trial, the 8-year EFS rate of CNS3 patients (n = 49) treated without cranial radiation therapy was 68%. The cumulative incidence of isolated CNS relapse for those patients was 9.4%.[
27 ][Level of evidence B4]
- On the SJCRH Total XV (TOTXV) study, patients with CNS3 status (N = 9) were treated without cranial radiation therapy (observed 5-year EFS rate, 43% ± 23%; OS rate, 71% ± 22%).[
- A meta-analysis of aggregated data from more than 16,000 patients treated between 1996 and 2007 by ten cooperative groups evaluated whether the use of cranial radiation therapy affected outcome in high-risk patient subsets.[
14 ]- In subgroup analyses of high-risk subsets, only those with CNS3 status at diagnosis appeared to benefit from cranial radiation therapy, with a significantly lower rate of CNS relapses (isolated/any) in irradiated patients. However, even within this subgroup, OS rates were similar with or without the use of radiation therapy.
Larger prospective studies will be necessary to fully elucidate the safety of omitting cranial radiation therapy in CNS3 patients.
CNS Therapy Options Under Clinical Evaluation
Information about NCI-supported clinical trials can be found on the
Toxicity of CNS-Directed Therapy
Toxic effects of CNS-directed therapy for childhood ALL can be acute and subacute or late developing. For more information, see the Late Effects of the Central Nervous System section in Late Effects of Treatment for Childhood Cancer.
Acute and subacute toxicities
The most common acute side effect associated with intrathecal chemotherapy alone is seizures. Up to 5% of nonirradiated patients with ALL treated with frequent doses of intrathecal chemotherapy will have at least one seizure during therapy.[
Patients with ALL who develop seizures during the course of treatment and who receive anticonvulsant therapy should not receive phenobarbital or phenytoin as anticonvulsant treatment, as these drugs may increase the clearance of some chemotherapeutic drugs and adversely affect treatment outcome.[
Late-developing toxicities
Late effects associated with CNS-directed therapies include subsequent neoplasms, neuroendocrine disturbances, leukoencephalopathy, and neurocognitive impairments.
Subsequent neoplasms are observed primarily in survivors who received cranial radiation therapy. Meningiomas are the most commonly observed second neoplasm and are most often of low malignant potential. However, high-grade lesions can also occur. In a SJCRH retrospective study of more than 1,290 patients with ALL who had never relapsed, the 30-year cumulative incidence of a subsequent neoplasm occurring in the CNS was 3%. Excluding meningiomas, the 30-year cumulative incidence was 1.17%.[
Neurocognitive impairments, which can range in severity and functional consequences, have been documented in long-term ALL survivors treated both with and without radiation therapy. In general, patients treated without cranial radiation therapy have less severe neurocognitive sequelae than irradiated patients, and the deficits that do develop represent relatively modest declines in a limited number of domains of neuropsychological functioning.[
Several studies have also evaluated the impact of other components of treatment on the development of late neurocognitive impairments. A comparison of neurocognitive outcomes of patients treated with methotrexate versus triple intrathecal chemotherapy showed no clinically meaningful difference.[
Evidence (neurocognitive late effects of cranial radiation):
- A SJCRH study of 567 adult long-term survivors of childhood ALL underwent neurocognitive testing (mean time from diagnosis, 26 years).[
40 ]- Patients treated with 24 Gy of cranial radiation therapy showed the highest rates of impairment. Up to one-third of these patients demonstrated impairments (defined as test scores 2 or more standard deviations below age-adjusted national norms) in attention, memory, processing speed, and executive function.
- Significantly fewer patients who had received 18 Gy of cranial radiation therapy demonstrated severe impairments compared with those who had received 24 Gy. In general, there was no significant difference in rates of impairment between nonirradiated survivors and those who received 18 Gy of cranial radiation therapy. However, the 18-Gy group was at increased risk of academic problems.
- In addition to being dose-related, the neurocognitive impact of cranial radiation therapy was also dependent on age at diagnosis, with higher frequency of impairments in patients diagnosed at a younger age.
- A study compared memory impairment in patients who received 18 Gy of cranial radiation therapy (n = 127) versus 24 Gy of cranial radiation therapy (n = 138).[
42 ]- Long-term survivors who received 24 Gy of cranial radiation therapy demonstrated significant impairments in immediate and delayed memory, compared with survivors who received 18 Gy.
- In a randomized trial comparing irradiated (at a dose of 18 Gy) and nonirradiated standard-risk ALL patients, the following was observed: [
34 ][Level of evidence A3]- Cognitive function for both groups (assessed at a median of 6 years postdiagnosis) was in the average range, with only subtle differences noted between the groups in cognitive skills.
- In a randomized trial, hyperfractionated radiation therapy (at a dose of 18 Gy) did not decrease neurological late effects when compared with conventionally fractionated radiation therapy. Cognitive function for both groups was not significantly impaired.[
43 ]
Evidence (neurocognitive late effects in nonirradiated patients):
- In the SJCRH long-term follow-up study of 567 adult long-term survivors, some nonirradiated patients also demonstrated neurocognitive impairments.[
40 ]- The age-adjusted mean test scores for nonirradiated patients were very similar to that of expected national norms. However, approximately 15% of the nonirradiated survivors participating in this study demonstrated impairments in some domains, including attention, memory, processing speed, and executive function.
- Despite the impairments noted on neurocognitive testing, overall, the educational attainment and employment status of the tested ALL survivors were similar to age- and sex-adjusted expected proportions using census data for the U.S. population.
- In a second study from SJCRH, patients enrolled on Total Study XV (which omitted cranial radiation therapy in all patients) underwent comprehensive neuropsychological assessments at induction, end of maintenance, and 2 years after completion of therapy.[
44 ]- Neurocognitive function was largely age appropriate 2 years after completing therapy, without evidence of excess impairment on measures of intellectual functioning, academic abilities, learning, and memory. Problems with sustained attention were observed at an increased frequency in this population compared with normative expectations.
- High-risk patients who received more intensive CNS-directed chemotherapy (including high-dose methotrexate and more doses of intrathecal chemotherapy) were at greater risk of difficulties in attention, processing speed, and academics.
- In a subsequent study from the SJCRH, 400 patients in the Total XVI study (enrolled between 2007 and 2017) underwent neurocognitive testing after the completion of therapy (approximately 3 years from diagnosis). None of the patients received cranial radiation, but the number of doses of triple intrathecal therapy (ITT) chemotherapy varied by risk group and CNS status. Patients with low-risk ALL were prescribed 13 to 21 doses, while patients with standard- and high-risk ALL were prescribed 16 to 27 doses.[
45 ]- Compared with age-normative expectations, the entire patient cohort exhibited neurocognitive impairments in multiple domains, including attention, working memory, executive function, fine motor skills, and adaptive skills.
- For patients with low-risk ALL, there was no correlation between the number of ITT doses (<20 vs. >21) and neurocognitive measures.
- For patients with standard- and high-risk ALL, those who received the highest number of ITT doses (>27) had an increased risk of impairment than those who received fewer doses, especially in working memory, attention, and fine motor skills.
- Patients with public or no insurance had worse neurocognitive outcomes than those with private insurance.
- Among patients with standard- and high-risk ALL, boys had a higher risk of neurocognitive impairments than girls, as did patients who were younger at diagnosis.
- The clinical manifestations of the neurocognitive impairments (if any) found by end-of-treatment neurocognitive testing, and their trajectories over time, were not evaluated in this study.
- In the COG AALL06N1 (NCT00437060) trial, patients with high-risk B-ALL who had been enrolled in the AALL0232 trial received a neurocognitive evaluation. These patients had been randomly assigned to receive either high-dose methotrexate with leucovorin rescue or escalating-dose IV methotrexate with pegasparaginase during the interim maintenance phase of therapy in the AALL0232 trial. A neurocognitive evaluation, which included an assessment of intellectual function (estimated intelligence quotient [IQ]), working memory, and processing speed, was conducted after the completion of therapy.[
46 ]- The method of methotrexate delivery was unrelated to differences in neurocognitive outcomes after controlling for ethnicity, race, age, gender, insurance status, and time off treatment.
- Survivors younger than 10 years at diagnosis had a statistically significantly lower estimated IQ and processing speed scores than older patients.
- Additionally, patients with public health insurance had significantly lower estimated IQ scores than participants with U.S. private or military insurance.
References:
- Richards S, Pui CH, Gayon P, et al.: Systematic review and meta-analysis of randomized trials of central nervous system directed therapy for childhood acute lymphoblastic leukemia. Pediatr Blood Cancer 60 (2): 185-95, 2013.
- Mahmoud HH, Rivera GK, Hancock ML, et al.: Low leukocyte counts with blast cells in cerebrospinal fluid of children with newly diagnosed acute lymphoblastic leukemia. N Engl J Med 329 (5): 314-9, 1993.
- Bürger B, Zimmermann M, Mann G, et al.: Diagnostic cerebrospinal fluid examination in children with acute lymphoblastic leukemia: significance of low leukocyte counts with blasts or traumatic lumbar puncture. J Clin Oncol 21 (2): 184-8, 2003.
- Gajjar A, Harrison PL, Sandlund JT, et al.: Traumatic lumbar puncture at diagnosis adversely affects outcome in childhood acute lymphoblastic leukemia. Blood 96 (10): 3381-4, 2000.
- Pullen J, Boyett J, Shuster J, et al.: Extended triple intrathecal chemotherapy trial for prevention of CNS relapse in good-risk and poor-risk patients with B-progenitor acute lymphoblastic leukemia: a Pediatric Oncology Group study. J Clin Oncol 11 (5): 839-49, 1993.
- Thyss A, Suciu S, Bertrand Y, et al.: Systemic effect of intrathecal methotrexate during the initial phase of treatment of childhood acute lymphoblastic leukemia. The European Organization for Research and Treatment of Cancer Children's Leukemia Cooperative Group. J Clin Oncol 15 (5): 1824-30, 1997.
- Rizzari C, Lanvers-Kaminsky C, Valsecchi MG, et al.: Asparagine levels in the cerebrospinal fluid of children with acute lymphoblastic leukemia treated with pegylated-asparaginase in the induction phase of the AIEOP-BFM ALL 2009 study. Haematologica 104 (9): 1812-1821, 2019.
- Bostrom BC, Sensel MR, Sather HN, et al.: Dexamethasone versus prednisone and daily oral versus weekly intravenous mercaptopurine for patients with standard-risk acute lymphoblastic leukemia: a report from the Children's Cancer Group. Blood 101 (10): 3809-17, 2003.
- Matloub Y, Bostrom BC, Hunger SP, et al.: Escalating intravenous methotrexate improves event-free survival in children with standard-risk acute lymphoblastic leukemia: a report from the Children's Oncology Group. Blood 118 (2): 243-51, 2011.
- Asselin BL, Devidas M, Wang C, et al.: Effectiveness of high-dose methotrexate in T-cell lymphoblastic leukemia and advanced-stage lymphoblastic lymphoma: a randomized study by the Children's Oncology Group (POG 9404). Blood 118 (4): 874-83, 2011.
- Pui CH, Howard SC: Current management and challenges of malignant disease in the CNS in paediatric leukaemia. Lancet Oncol 9 (3): 257-68, 2008.
- Pui CH, Campana D, Pei D, et al.: Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med 360 (26): 2730-41, 2009.
- Veerman AJ, Kamps WA, van den Berg H, et al.: Dexamethasone-based therapy for childhood acute lymphoblastic leukaemia: results of the prospective Dutch Childhood Oncology Group (DCOG) protocol ALL-9 (1997-2004). Lancet Oncol 10 (10): 957-66, 2009.
- Vora A, Andreano A, Pui CH, et al.: Influence of Cranial Radiotherapy on Outcome in Children With Acute Lymphoblastic Leukemia Treated With Contemporary Therapy. J Clin Oncol 34 (9): 919-26, 2016.
- Pui CH, Sandlund JT, Pei D, et al.: Improved outcome for children with acute lymphoblastic leukemia: results of Total Therapy Study XIIIB at St Jude Children's Research Hospital. Blood 104 (9): 2690-6, 2004.
- Tubergen DG, Gilchrist GS, O'Brien RT, et al.: Prevention of CNS disease in intermediate-risk acute lymphoblastic leukemia: comparison of cranial radiation and intrathecal methotrexate and the importance of systemic therapy: a Childrens Cancer Group report. J Clin Oncol 11 (3): 520-6, 1993.
- Conter V, Aricò M, Valsecchi MG, et al.: Extended intrathecal methotrexate may replace cranial irradiation for prevention of CNS relapse in children with intermediate-risk acute lymphoblastic leukemia treated with Berlin-Frankfurt-Münster-based intensive chemotherapy. The Associazione Italiana di Ematologia ed Oncologia Pediatrica. J Clin Oncol 13 (10): 2497-502, 1995.
- Möricke A, Reiter A, Zimmermann M, et al.: Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood 111 (9): 4477-89, 2008.
- Clarke M, Gaynon P, Hann I, et al.: CNS-directed therapy for childhood acute lymphoblastic leukemia: Childhood ALL Collaborative Group overview of 43 randomized trials. J Clin Oncol 21 (9): 1798-809, 2003.
- Moghrabi A, Levy DE, Asselin B, et al.: Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95-01 for children with acute lymphoblastic leukemia. Blood 109 (3): 896-904, 2007.
- Matloub Y, Lindemulder S, Gaynon PS, et al.: Intrathecal triple therapy decreases central nervous system relapse but fails to improve event-free survival when compared with intrathecal methotrexate: results of the Children's Cancer Group (CCG) 1952 study for standard-risk acute lymphoblastic leukemia, reported by the Children's Oncology Group. Blood 108 (4): 1165-73, 2006.
- Mitchell CD, Richards SM, Kinsey SE, et al.: Benefit of dexamethasone compared with prednisolone for childhood acute lymphoblastic leukaemia: results of the UK Medical Research Council ALL97 randomized trial. Br J Haematol 129 (6): 734-45, 2005.
- Vrooman LM, Stevenson KE, Supko JG, et al.: Postinduction dexamethasone and individualized dosing of Escherichia Coli L-asparaginase each improve outcome of children and adolescents with newly diagnosed acute lymphoblastic leukemia: results from a randomized study--Dana-Farber Cancer Institute ALL Consortium Protocol 00-01. J Clin Oncol 31 (9): 1202-10, 2013.
- Kadan-Lottick NS, Brouwers P, Breiger D, et al.: Comparison of neurocognitive functioning in children previously randomly assigned to intrathecal methotrexate compared with triple intrathecal therapy for the treatment of childhood acute lymphoblastic leukemia. J Clin Oncol 27 (35): 5986-92, 2009.
- Salzer WL, Burke MJ, Devidas M, et al.: Impact of Intrathecal Triple Therapy Versus Intrathecal Methotrexate on Disease-Free Survival for High-Risk B-Lymphoblastic Leukemia: Children's Oncology Group Study AALL1131. J Clin Oncol 38 (23): 2628-2638, 2020.
- Schrappe M, Reiter A, Ludwig WD, et al.: Improved outcome in childhood acute lymphoblastic leukemia despite reduced use of anthracyclines and cranial radiotherapy: results of trial ALL-BFM 90. German-Austrian-Swiss ALL-BFM Study Group. Blood 95 (11): 3310-22, 2000.
- Sirvent N, Suciu S, Rialland X, et al.: Prognostic significance of the initial cerebro-spinal fluid (CSF) involvement of children with acute lymphoblastic leukaemia (ALL) treated without cranial irradiation: results of European Organization for Research and Treatment of Cancer (EORTC) Children Leukemia Group study 58881. Eur J Cancer 47 (2): 239-47, 2011.
- Piette C, Suciu S, Bertrand Y, et al.: Long-term outcome evaluation of medium/high risk acute lymphoblastic leukaemia children treated with or without cranial radiotherapy in the EORTC 58832 randomized study. Br J Haematol 189 (2): 351-362, 2020.
- Mahoney DH, Shuster JJ, Nitschke R, et al.: Acute neurotoxicity in children with B-precursor acute lymphoid leukemia: an association with intermediate-dose intravenous methotrexate and intrathecal triple therapy--a Pediatric Oncology Group study. J Clin Oncol 16 (5): 1712-22, 1998.
- Bhojwani D, Sabin ND, Pei D, et al.: Methotrexate-induced neurotoxicity and leukoencephalopathy in childhood acute lymphoblastic leukemia. J Clin Oncol 32 (9): 949-59, 2014.
- Relling MV, Pui CH, Sandlund JT, et al.: Adverse effect of anticonvulsants on efficacy of chemotherapy for acute lymphoblastic leukaemia. Lancet 356 (9226): 285-90, 2000.
- Hijiya N, Hudson MM, Lensing S, et al.: Cumulative incidence of secondary neoplasms as a first event after childhood acute lymphoblastic leukemia. JAMA 297 (11): 1207-15, 2007.
- Casey DL, Vogelius IR, Brodin NP, et al.: Risk of Subsequent Neoplasms in Childhood Cancer Survivors After Radiation Therapy: A PENTEC Comprehensive Review. Int J Radiat Oncol Biol Phys 119 (2): 640-654, 2024.
- Waber DP, Turek J, Catania L, et al.: Neuropsychological outcomes from a randomized trial of triple intrathecal chemotherapy compared with 18 Gy cranial radiation as CNS treatment in acute lymphoblastic leukemia: findings from Dana-Farber Cancer Institute ALL Consortium Protocol 95-01. J Clin Oncol 25 (31): 4914-21, 2007.
- Jansen NC, Kingma A, Schuitema A, et al.: Neuropsychological outcome in chemotherapy-only-treated children with acute lymphoblastic leukemia. J Clin Oncol 26 (18): 3025-30, 2008.
- Espy KA, Moore IM, Kaufmann PM, et al.: Chemotherapeutic CNS prophylaxis and neuropsychologic change in children with acute lymphoblastic leukemia: a prospective study. J Pediatr Psychol 26 (1): 1-9, 2001 Jan-Feb.
- Copeland DR, Moore BD, Francis DJ, et al.: Neuropsychologic effects of chemotherapy on children with cancer: a longitudinal study. J Clin Oncol 14 (10): 2826-35, 1996.
- von der Weid N, Mosimann I, Hirt A, et al.: Intellectual outcome in children and adolescents with acute lymphoblastic leukaemia treated with chemotherapy alone: age- and sex-related differences. Eur J Cancer 39 (3): 359-65, 2003.
- Waber DP, Carpentieri SC, Klar N, et al.: Cognitive sequelae in children treated for acute lymphoblastic leukemia with dexamethasone or prednisone. J Pediatr Hematol Oncol 22 (3): 206-13, 2000 May-Jun.
- Krull KR, Brinkman TM, Li C, et al.: Neurocognitive outcomes decades after treatment for childhood acute lymphoblastic leukemia: a report from the St Jude lifetime cohort study. J Clin Oncol 31 (35): 4407-15, 2013.
- Kadan-Lottick NS, Brouwers P, Breiger D, et al.: A comparison of neurocognitive functioning in children previously randomized to dexamethasone or prednisone in the treatment of childhood acute lymphoblastic leukemia. Blood 114 (9): 1746-52, 2009.
- Armstrong GT, Reddick WE, Petersen RC, et al.: Evaluation of memory impairment in aging adult survivors of childhood acute lymphoblastic leukemia treated with cranial radiotherapy. J Natl Cancer Inst 105 (12): 899-907, 2013.
- Waber DP, Silverman LB, Catania L, et al.: Outcomes of a randomized trial of hyperfractionated cranial radiation therapy for treatment of high-risk acute lymphoblastic leukemia: therapeutic efficacy and neurotoxicity. J Clin Oncol 22 (13): 2701-7, 2004.
- Jacola LM, Krull KR, Pui CH, et al.: Longitudinal Assessment of Neurocognitive Outcomes in Survivors of Childhood Acute Lymphoblastic Leukemia Treated on a Contemporary Chemotherapy Protocol. J Clin Oncol 34 (11): 1239-47, 2016.
- Jacola LM, Conklin HM, Krull KR, et al.: The Impact of Intensified CNS-Directed Therapy on Neurocognitive Outcomes in Survivors of Childhood Acute Lymphoblastic Leukemia Treated Without Cranial Irradiation. J Clin Oncol 40 (36): 4218-4227, 2022.
- Hardy KK, Embry L, Kairalla JA, et al.: Neurocognitive Functioning of Children Treated for High-Risk B-Acute Lymphoblastic Leukemia Randomly Assigned to Different Methotrexate and Corticosteroid Treatment Strategies: A Report From the Children's Oncology Group. J Clin Oncol 35 (23): 2700-2707, 2017.
Postinduction Treatment for Specific ALL Subgroups
T-ALL
Historically, patients with T-acute lymphoblastic leukemia (ALL) have had a worse prognosis than children with B-ALL. In a review of a large number of patients treated on Children's Oncology Group (COG) trials over a 15-year period, T-cell immunophenotype still proved to be a negative prognostic factor on multivariate analysis.[
Treatment options for T-ALL
Treatment options for T-ALL include the following:
- Chemotherapy with or without prophylactic cranial radiation therapy.
Evidence (chemotherapy and prophylactic cranial radiation therapy):
- Protocols of the former Pediatric Oncology Group (POG) treated children with T-ALL differently from children with B-ALL. On the POG-9404 protocol, patients with T-ALL were treated using a regimen based on the DFCI 87-001 protocol, which included multiple doses of L-asparaginase and doxorubicin during postinduction therapy, as well as prophylactic cranial radiation therapy. Patients were randomly assigned to either the high-dose methotrexate arm or the control arm.[
4 ]- Patients randomly assigned to receive high-dose methotrexate achieved a superior outcome (10-year EFS rates were 78% with high-dose methotrexate vs. 68% for the control arm).[
5 ,6 ]
- Patients randomly assigned to receive high-dose methotrexate achieved a superior outcome (10-year EFS rates were 78% with high-dose methotrexate vs. 68% for the control arm).[
- In the POG-9404 study, patients were also randomly assigned to receive doxorubicin with or without dexrazoxane to determine the efficacy of dexrazoxane in preventing late cardiac mortality.[
7 ][Level of evidence B1]- There was no difference in EFS between patients with T-ALL who were treated with dexrazoxane and patients who were not treated with dexrazoxane (cumulative doxorubicin dose, 360 mg/m2).[
7 ] - The frequency of grade 3 and grade 4 toxicities that occurred during therapy was similar between the randomized groups, and there was no difference in cumulative incidence of second malignant neoplasms. Three years after initial diagnosis, left ventricular shortening fraction and left ventricular wall thickness were both significantly worse in patients who received doxorubicin alone than in patients who received dexrazoxane, indicating that dexrazoxane was cardioprotective.[
7 ] - With combined data from three COG trials that randomized dexrazoxane with doxorubicin therapy (P9404, P9425, and P9426) and had a median follow-up of 12.6 years, dexrazoxane did not appear to compromise long-term survival.[
8 ][Level of evidence A1]
- There was no difference in EFS between patients with T-ALL who were treated with dexrazoxane and patients who were not treated with dexrazoxane (cumulative doxorubicin dose, 360 mg/m2).[
- On protocols of the former Children's Cancer Group (CCG), children with T-ALL received the same treatment regimens as did children with B-ALL. Protocol and treatment assignment were based on the patients' clinical characteristics (e.g., age and white blood cell [WBC] count) and the disease response to initial therapy. Most children with T-ALL met National Cancer Institute (NCI) high-risk criteria.
- Results from the CCG-1961 trial for high-risk ALL, which included patients with T-ALL, showed that an augmented Berlin-Frankfurt-Münster (BFM) regimen with a single delayed intensification course produced the best results for patients with morphological rapid response to initial induction therapy (estimated 5-year EFS rate, 83%).[
9 ,10 ] With this approach, patients with a presenting WBC count greater than 200,000 had similar outcomes to those with a WBC count of less than 200,000.[11 ][Level of evidence B1] - Overall results from POG-9404 and CCG-1961 were similar, although POG-9404 used a higher cumulative dose of anthracyclines and cranial radiation therapy for every patient, while CCG-1961 used cranial radiation therapy only for patients with slow morphological response.[
10 ,5 ] - Among children with NCI standard-risk T-ALL treated on the CCG-1952, COG-1991, and POG-9404 trials, the 7-year EFS rates were comparable with those of patients treated with the CCG regimens that used significantly less anthracycline in a less intensive chemotherapy backbone without the prophylactic cranial irradiation included in POG-9404.[
12 ] However, the patients with NCI standard-risk T-ALL had an inferior EFS and OS compared with patients with NCI standard-risk B-ALL treated with the same regimens on the CCG-1952 and COG-1991 trials.[12 ]
- Results from the CCG-1961 trial for high-risk ALL, which included patients with T-ALL, showed that an augmented Berlin-Frankfurt-Münster (BFM) regimen with a single delayed intensification course produced the best results for patients with morphological rapid response to initial induction therapy (estimated 5-year EFS rate, 83%).[
- In the COG, children with T-ALL are not treated on the same protocols as children with B-ALL.
- Pilot studies from the COG have demonstrated the feasibility of incorporating nelarabine (a nucleoside analog with demonstrated activity in patients with relapsed and refractory T-cell lymphoblastic disease) in the context of a BFM regimen for patients with newly diagnosed T-ALL.[
13 ,14 ,15 ]- The pilot study showed a 5-year EFS rate of 73% for all patients who received nelarabine and 69% for those patients with a slow early response.[
16 ]
- The pilot study showed a 5-year EFS rate of 73% for all patients who received nelarabine and 69% for those patients with a slow early response.[
- The COG AALL0434 (NCT00408005) trial enrolled 1,562 evaluable patients aged 1 to 31 years with T-ALL. Patients received an augmented BFM regimen and were randomly assigned to receive an interim maintenance phase with either high-dose methotrexate with leucovorin rescue or escalating-dose methotrexate without leucovorin but with pegaspargase.[
3 ] Intermediate-risk and high-risk patients were also randomly assigned to receive either six courses of nelarabine during postinduction treatment or no nelarabine.[17 ] Nearly all patients received either prophylactic (12 Gy) or therapeutic (18 Gy) cranial irradiation. Only 10% of patients considered to be low risk were not irradiated. Patients assigned to the escalating-dose methotrexate arm received cranial radiation therapy earlier than did patients assigned to the high-dose methotrexate arm (week 8 vs. week 26). Patients on the escalating-dose methotrexate arm also received two additional doses of pegaspargase. Results were as follows:[3 ,17 ]- The overall 5-year EFS rate was 83.8%, and the OS rate was 89.5%.
- Results indicated a better disease-free survival (DFS) for patients who were randomly assigned to the escalating-dose methotrexate arm (5-year DFS rate, 91.5%) than for patients randomly assigned to the high-dose methotrexate arm (5-year DFS rate, 85.3%; P = .005).
- For intermediate-risk and high-risk patients, treatment with nelarabine was associated with a superior outcome (5-year DFS rate, 88.2% with nelarabine vs. 82.1% without nelarabine; P = .029). The 5-year cumulative incidence of central nervous system (CNS) relapse was significantly lower for patients treated with nelarabine (1.3% vs. 6.9% in the no-nelarabine arm).
- The best outcome for intermediate-risk and high-risk patients was observed in those randomly assigned to both the escalating-dose methotrexate and nelarabine arms (5-year DFS rate, 91.4%). The worst outcome was observed in patients randomly assigned to receive high-dose methotrexate and no nelarabine (5-year DFS rate, 78.1%).
- For patients with CNS3 disease, all of whom were assigned to receive high-dose methotrexate and 18 Gy of cranial radiation therapy, nelarabine was associated with significantly superior DFS.[
18 ] - Patients with initial induction failure (M3 marrow at day 29, n = 43) were nonrandomly assigned to receive high-dose methotrexate and nelarabine; 20 of these patients were removed from the protocol therapy to undergo allogeneic hematopoietic stem cell transplant (HSCT) in first complete remission (CR). The overall 5-year EFS rate for induction-failure patients was 53%, with no difference in outcomes between HSCT and chemotherapy.
- In the successor COG AALL1231 (NCT02112916) trial for T-ALL, patients were randomly assigned to receive or not receive the proteasome inhibitor bortezomib during induction and delayed intensification phases. Patients with initial induction failure (M3 marrow at day 29) or high MRD (≥0.1%) at end of consolidation were nonrandomly assigned to receive three additional intensified chemotherapy blocks postconsolidation. Other nonrandomized changes to treatment included the use of dexamethasone during induction (instead of prednisone) and omission of cranial radiation therapy for all but the very high-risk patients. Because the results of AALL0434 trial were not known at the time that AALL1231 opened, nelarabine was not included in the chemotherapy backbone. The trial was designed to enroll 1,200 patients but closed to accrual early (after 824 evaluable patients had enrolled) once the results of the nelarabine randomization on AALL0434 were released.[
19 ]- Comparing patients with T-ALL assigned to receive bortezomib versus patients who did not receive bortezomib, there was no difference in the 4-year EFS rate (82.9% vs. 81.5%; P = .396) or the 5-year OS rate (87.9% vs. 88.3%; P = .469).
- Subset analyses were performed comparing similar patients with T-ALL scheduled to receive 12 Gy cranial radiation therapy on the AALL0434 trial (90.8% of patients), but not on the AALL1231 trial (9.5% of patients). Excluding patients who received nelarabine (AALL0434) or bortezomib (AALL1231), the 4-year EFS (P = .412), OS (P = .600), cumulative incidence of CNS relapse (P = .456), and overall relapse (P = .836) rates were not significantly different between the studies, indicating that the omission of cranial radiation (in the context of the AALL1231 chemotherapy backbone) did not adversely impact outcome.
- While the 4-year EFS rates were similar between the AALL1231 and AALL0434 trials (P = .131), the 4-year OS rate on the AALL1231 trial was inferior to that on the AALL0434 trial (87% vs. 90%, P = .006).
- While the cumulative incidence of relapse was similar in both studies (P = .562), there was a higher rate of treatment-related mortality on the AALL1231 trial, compared with the AALL0434 trial (6.1% vs. 2.0%). Rates of treatment-related mortality were higher on the AALL1231 trial during both induction (1.5% vs. 0.4%, P = .002) and postinduction treatment phases (3.9% vs. 2.1%, P = .008).
- Despite intensification of the chemotherapy backbone, outcome was poor for patients with T-ALL assigned to the very high-risk group (5.2% of patients). The 4-year EFS rate was 31.3% for patients on the no-bortezomib arm, compared with 7.8% for patients on the bortezomib arm (P = .033).
- Pilot studies from the COG have demonstrated the feasibility of incorporating nelarabine (a nucleoside analog with demonstrated activity in patients with relapsed and refractory T-cell lymphoblastic disease) in the context of a BFM regimen for patients with newly diagnosed T-ALL.[
The use of prophylactic cranial radiation therapy in the treatment of patients with T-ALL is declining. Some groups, such as St. Jude Children's Research Hospital (SJCRH) and the Dutch Childhood Oncology Group (DCOG), do not use cranial radiation therapy in first-line treatment of ALL. Other groups, such as DFCI, COG, and BFM, are now limiting radiation therapy to patients with very high-risk features or CNS3 disease.
Treatment options under clinical evaluation for T-ALL
Information about NCI-supported clinical trials can be found on the
Current Clinical Trials
Use our
Infants With ALL
Infant ALL is uncommon, representing approximately 2% to 4% of cases of childhood ALL.[
Infants diagnosed within the first few months of life have a particularly poor outcome. In one study, patients diagnosed within 1 month of birth had a 2-year OS rate of 20%.[
For infants with KMT2A gene rearrangements, the EFS rates at 4 to 5 years continue to be in the 35% range.[
- Younger age at diagnosis (≤90–180 days).
- Extremely high presenting leukocyte count (≥200,000–300,000/μL).
- Poor early response, as reflected by a poor response to a prednisone prophase or high levels of minimal residual disease (MRD) at the end of induction and consolidation phases of treatment.
In one report, any CNS involvement at diagnosis (CNS2, CNS3, or traumatic lumbar puncture with blasts) was also found to be an independent predictor of adverse outcome in infants with KMT2A-rearranged ALL.[
In addition to having a significantly higher relapse rate than older children with ALL, infant patients more frequently present with a higher acuity. In a large retrospective study, infants with ALL were more likely to present with multisystem organ failure than noninfants (12% and 1%). Infants also had greater requirements for blood products, diuretics, supplemental oxygen, and mechanical ventilation during induction, compared with noninfants.[
Infants are also at higher risk of developing treatment-related toxicity, especially infection. With current treatment approaches for this population, treatment-related mortality has been reported to occur in about 10% of infants, a rate that is much higher than the rate in older children with ALL.[
Treatment options for infants withKMT2Arearrangements
Infants with KMT2A gene rearrangements are generally treated on intensified chemotherapy regimens using agents not typically incorporated into frontline therapy for older children with ALL. However, despite these intensified approaches, EFS rates remain poor for these patients.
Evidence (intensified chemotherapy regimens for infants with KMT2A rearrangements):
- The international Interfant-99 trial used a cytarabine-intensive chemotherapy regimen, with increased exposure to both low- and high-dose cytarabine during the first few months of therapy.[
22 ]- The 5-year EFS rate was 37% for infants with KMT2A rearrangements.
- The COG tested intensification of therapy with a regimen that included multiple doses of high-dose methotrexate, cyclophosphamide, and etoposide.[
21 ]- The 5-year EFS rate was 34% for infants with KMT2A rearrangements.
- On the COG P9407 (NCT00002756) trial, infants were treated with a shortened (46-week) intensive chemotherapy regimen.[
23 ][Level of evidence B4]- The 5-year EFS rate was 36% for infants with KMT2A rearrangements.
- The international Interfant-06 study tested whether acute myeloid leukemia (AML)-style consolidation chemotherapy was superior to ALL-style chemotherapy.[
29 ][Level of evidence B4]- The 6-year EFS rate was 46.1%, and the OS rate was 58.2%. These rates were not statistically different from the rates observed in the predecessor Interfant-99 protocol.
- For infants with KMT2A rearrangements, the 6-year EFS rate was 36.4%, with no significant difference between the AML and ALL approach.
- In a subsequent analysis of the subset of KMT2A-rearranged patients in whom MRD was evaluated, both end-of-induction (EOI) and end-of-consolidation (EOC) MRD were strongly predictive of outcome. Patients who were EOI MRD positive, but became EOC MRD negative, had similar outcomes to those who were EOI MRD negative (6-year DFS rates, 65.7% and 72.0%, respectively). Patients with high EOC MRD had dismal outcomes (6-year DFS rate, 13.1%). Patients with negative EOI MRD had a higher 6-year DFS rate when treated with the ALL approach (78.2%) than did patients who were treated with the AML approach (45%). However, patients with high EOI MRD (≥5 × 10-4) who were treated with an AML approach had a superior 6-year DFS rate (45.9%) when compared with patients who were treated with the ALL approach (23.2%).[
33 ][Level of evidence B1]
- In a follow-up pilot trial conducted by the Interfant study group, 30 infants with KMT2A-rearranged ALL were treated using the Interfant-06 chemotherapy backbone, with the addition of one 28-day course of blinatumomab after the induction phase.[
34 ]- Blinatumomab was well tolerated, without any excessive or unexpected toxicity in these infant patients.
- With short median follow-up (26.3 months), the 2-year DFS rate was 81.6% (95% CI, 60.8%–92.0%), and the 2-year OS rate was 93.3% (95% CI, 75.9%–98.3%).
- These outcomes were superior to the 2-year DFS and OS rates of historical controls who were treated in the Interfant-06 protocol (DFS and OS rates, 49.4% and 65.8%, respectively).
- On the MLL-10 trial conducted by the Japanese Pediatric Leukemia/Lymphoma Study Group (JPLSG), infants with KMT2A rearrangements were treated with an intensified chemotherapy backbone that included multiple phases using high-dose methotrexate, cyclophosphamide, etoposide, and high-dose cytarabine. Patients classified as high-risk on the basis of age and CNS status (75% of KMT2A-rearranged patients) were allocated to HSCT in first CR.[
24 ]- The 5-year EFS rate was 66%, and the 5-year OS rate was 82%.
- In the COG AALL0631 (NCT00557193) trial, infants with KMT2A-rearranged ALL were assigned to receive an intensive chemotherapy regimen with or without lestaurtinib, an FLT3 inhibitor (administered during postinduction treatment phases).[
27 ]- The overall 5-year EFS rate for all participants was 34%, and the 5-year OS rate was 41%.
- There was no difference in outcome between patients on the lestaurtinib arm versus those who received chemotherapy only.
Exploratory studies were conducted to evaluate the impact of sufficient lestaurtinib blood levels to achieve FLT3 inhibition and to evaluate the impact of ex vivo sensitivity of leukemia cells to lestaurtinib.
- Only 38% of lestaurtinib-treated patients demonstrated in vivo FLT3 inhibition. This subset of patients appeared to have a better outcome than other patients on the lestaurtinib arm, as did those whose blasts demonstrated ex vivo sensitivity to FLT3 inhibition.
The role of allogeneic HSCT during first remission in infants with KMT2A gene rearrangements remains controversial.
Evidence (allogeneic HSCT in first remission for infants with KMT2A rearrangements):
- On a trial conducted by the JPLSG between 1998 and 2002, all infants with KMT2A rearrangements were intended to proceed to allogeneic HSCT from the best available donor (related, unrelated, or umbilical cord) 3 to 5 months after diagnosis.[
35 ]- The 3-year EFS rate for all enrolled infants was 44%. This outcome resulted, in part, from the high frequency of early relapses, even with intensive chemotherapy. Of the 41 infants with KMT2A rearrangements on that study who achieved CR, 11 infants (27%) relapsed before proceeding to transplant.
- In a follow-up trial conducted by the JPLSG between 2011 and 2015, allocation to HSCT in first CR was restricted to the patients with KMT2A rearrangements and classified as high risk (age <6 months and/or CNS3 status at diagnosis).[
24 ]- Of the 56 patients that were classified as high risk (75% of all KMT2A-rearranged patients enrolled on the trial), 49 achieved CR, and 38 underwent HSCT.
- In an intent-to-treat analysis of all high-risk patients enrolled on the trial, the 5-year EFS rate was 56.6%.
- For the subset of patients who also met Interfant-06 criteria for high risk (age <6 months and WBC >300,000/µL or a prednisolone poor responder), the 5-year EFS rate was 45.2%.
- In a COG report that included 189 infants treated on CCG or POG infant ALL protocols between 1996 and 2000, there was no difference in EFS between patients who underwent HSCT in first CR and those who received chemotherapy alone.[
36 ] - The Interfant clinical trials group, after adjusting for waiting time to transplant, also did not observe any difference in DFS in high-risk infants (defined by prednisone response) with KMT2A rearrangements treated on the Interfant-99 trial with either allogeneic HSCT in first CR or chemotherapy alone.[
22 ]- In a subset analysis from the same trial, allogeneic HSCT in first remission was associated with a significantly better DFS for infants with KMT2A rearrangements who were younger than 6 months at diagnosis and had either a poor prednisone response at day 8 or leukocyte counts of at least 300,000/µL.[
37 ] In this subset, HSCT in first remission was associated with a 64% reduction in the risk of failure resulting from relapse or death compared with chemotherapy alone.
- In a subset analysis from the same trial, allogeneic HSCT in first remission was associated with a significantly better DFS for infants with KMT2A rearrangements who were younger than 6 months at diagnosis and had either a poor prednisone response at day 8 or leukocyte counts of at least 300,000/µL.[
- On the Interfant-06 study, infants considered to be high risk (all of the following: KMT2A rearrangements, age <6 months, and WBC ≥300,000/μL) were considered eligible for allogeneic HSCT in first CR.[
29 ][Level of evidence B4]- About one-half of the high-risk patients did not proceed to transplant in the first CR primarily because of early relapse.
- The 6-year EFS rate of the entire high-risk group was 21%.
- For the highly-selected population who underwent HSCT, the 4-year DFS rate was 44%.
For infants with ALL who undergo transplant in first CR, outcomes appear to be similar with non–total-body irradiation (TBI) regimens and TBI-based regimens.[
Treatment options for infants withoutKMT2Arearrangements
The optimal treatment for infants without KMT2A rearrangements also remains unclear, in part because of the paucity of data on the use of standard ALL regimens used in older children.
- On the Interfant-99 trial, patients without KMT2A rearrangements achieved a relatively favorable outcome with the cytarabine-intensive treatment regimen (4-year EFS rate, 74%).[
22 ] - The COG P9407 (NCT00002756) trial of intensified chemotherapy reported a 5-year EFS rate of 70% in infants without the KMT2A rearrangement.[
23 ][Level of evidence B4] - A favorable outcome for this subset of patients was obtained in a Japanese study using therapy comparable to that used to treat older children with ALL.[
26 ] However, that study was limited by small numbers (n = 22) and a highly unusual sex distribution (91% males). - On the Interfant-06 study, the 6-year EFS rate for infants without KMT2A rearrangements was 73.9%, and the OS rate was 87.2%.[
39 ]; [29 ][Level of evidence B4] - On the COG AALL0631 (NCT00557193) study, infants without KMT2A rearrangements received the same intensified chemotherapy backbone as those with the KMT2A rearrangement.[
40 ]- The 5-year EFS rate was 87.3% (± 4.7%), and the 5-year OS rate was 93.6% (± 3.5%) for 64 infants without KMT2A rearrangements.
Treatment options under clinical evaluation for infants with ALL
Information about NCI-supported clinical trials can be found on the
Adolescents and Young Adults With ALL
Adolescents and young adults with ALL have been recognized as high risk for decades. Outcomes for this age group are inferior in almost all studies of treatment compared with children younger than 10 years.[
- T-cell immunophenotype.
- BCR::ABL1 and BCR::ABL1-like disease.
- Lower incidence of favorable cytogenetic abnormalities.
In addition to more frequent adverse prognostic factors, patients in this age group have higher rates of treatment-related mortality [
Treatment options for adolescents and young adults with ALL
Studies from the United States and France were among the first to identify the difference in outcome based on treatment regimens.[
Given the relatively favorable outcome that can be obtained in these patients with chemotherapy regimens used for high-risk pediatric ALL, there is no role for the routine use of allogeneic HSCT for adolescents and young adults with ALL in first remission.[
Evidence (use of a pediatric treatment regimen for adolescents and young adults with ALL):
- The CALGB-10403 (NCT00558519) trial prospectively studied the feasibility and efficacy of using a pediatric treatment regimen (administered by medical oncologists) for adolescent and young adult patients with newly diagnosed ALL. Of the 318 patients enrolled, 295 were eligible and evaluable for response. The median age was 24 years (range, 17–39 years).[
55 ,57 ]- Use of the pediatric regimen (from the COG AALL0232 study, which included interim maintenance phases with escalating doses of methotrexate without leucovorin followed by asparaginase) was deemed safe, and the overall treatment-related mortality was 3%.
- The median EFS was 78.1 months, which is more than double the historical control of 30 months. The 3-year EFS rate was 59%, and the median OS was not reached. The estimated 3-year OS rate was 73%.
- Pretreatment risk factors associated with a worse outcome were obesity and the presence of the BCR::ABL1-like expression signature. Of the evaluable patients, 31% had a BCR::ABL1-like signature. These patients had a significantly worse outcome, with a 3-year EFS rate of 42%, compared with an EFS rate of 69% for patients without BCR::ABL1-like ALL (hazard ratio, 2.06; log-rank P = .008).
- End-induction MRD was a significant predictor of outcome. In the subset of patients evaluable for MRD, 44% had absence of detectable end-induction MRD using an Ig-TCR PCR-MRD assay (sensitivity of detection of 1 in 104 to 105). These patients had a 3-year DFS rate of 85%, compared with a 3-year DFS rate of 54% for those with detectable end-induction MRD (P = .001).
- In a study of patients aged 16 to 39 years, the outcomes of those who received postremission, standard, pediatric-inspired chemotherapy per the CALGB-10403 protocol were compared with the outcomes of patients who received myeloablative allogeneic HSCT. Patients who received chemotherapy had superior OS, DFS, and nonrelapse mortality rates.[
58 ][Level of evidence B4]
- Investigators reported on 197 patients aged 16 to 21 years treated on the CCG study (a pediatric ALL regimen) compared with 124 adolescents and young adults treated on the Cancer and Leukemia Group B (CALGB) study (an adult ALL regimen).[
47 ]- For the patients treated with a pediatric ALL regimen, the 7-year EFS rate was 63%.
- For the patients treated with an adult ALL regimen, the 7-year EFS rate was 34%.
- In a Canadian population-based cohort study, the effect of adapting pediatric protocols for adolescent and young adult patients with ALL was determined over a 20-year period.[
59 ]- The 5-year EFS rate was 72% for adolescent and young adult patients treated at pediatric centers, compared with 56% for adolescent and young adult patients treated at adult centers (P = .03).
- In the most recent period (2006–2011), the outcome of adolescent and young adult patients treated at adult centers with pediatric protocols was superior to those treated with adult protocols (EFS rate, 72% vs. 60%), but inferior to adolescent and young adult patients treated at pediatric centers (EFS rate, 81%; P = .02).
- The authors conclude that besides protocol therapy, there may be other differences between adult and pediatric centers that may explain the disparate outcomes.
The reason that adolescents and young adults achieve superior outcomes with pediatric regimens is not known, although possible explanations include the following:[
- Treatment setting (i.e., site experience in treating ALL).
- Adherence to protocol therapy.[
46 ] - The components of protocol therapy.
| Site and Study Group | Adolescent and Young Adult Patients (No.) | Median age (y) | Survival (%) |
|---|---|---|---|
| ALL = acute lymphoblastic leukemia; EFS = event-free survival; OS = overall survival. | |||
| AIEOP = Associazione Italiana di Ematologia e Oncologia Pediatrica; CALGB = Cancer and Leukemia Group B; CCG = Children's Cancer Group; DCOG = Dutch Childhood Oncology Group; FRALLE = French Acute Lymphoblastic Leukaemia Study Group; GIMEMA = Gruppo Italiano Malattie EMatologiche dell'Adulto; HOVON = Dutch-Belgian Hemato-Oncology Cooperative Group; LALA = France-Belgium Group for Lymphoblastic Acute Leukemia in Adults; MRC = Medical Research Council (United Kingdom); NOPHO = Nordic Society for Pediatric Hematology and Oncology; UKALL = United Kingdom Acute Lymphoblastic Leukaemia. | |||
| United States[ |
|||
| CCG (Pediatric) | 197 | 16 | 67, OS 7 y |
| CALGB (Adult) | 124 | 19 | 46 |
| France[ |
|||
| FRALLE 93 (Pediatric) | 77 | 16 | 67 EFS |
| LALA 94 | 100 | 18 | 41 |
| Italy[ |
|||
| AIEOP (Pediatric) | 150 | 15 | 80, OS 2 y |
| GIMEMA (Adult) | 95 | 16 | 71 |
| Netherlands[ |
|||
| DCOG (Pediatric) | 47 | 12 | 71 EFS |
| HOVON | 44 | 20 | 38 |
| Sweden[ |
|||
| NOPHO 92 (Pediatric) | 36 | 16 | 74, OS 5 y |
| Adult ALL | 99 | 18 | 39 |
| United Kingdom[ |
|||
| MRC ALL (Pediatric) | 61 | 15–17 | 71, OS 5 y |
| UKALL XII (Adult) | 67 | 15–17 | 56 |
| UKALL 2003[ |
229 | 16–24 | 72 EFS |
Osteonecrosis
Adolescents with ALL appear to be at higher risk than younger children for developing therapy-related complications, including osteonecrosis, deep venous thromboses, and pancreatitis.[
The weight-bearing joints are affected in 95% of patients who develop osteonecrosis and operative interventions were needed for management of symptoms and impaired mobility in more than 40% of cases. Most cases are diagnosed within the first 2 years of therapy and the symptoms are often recognized during maintenance.
Evidence (osteonecrosis):
- In the CCG-1961 high-risk ALL study, alternate-week dosing of dexamethasone was compared with standard continuous dexamethasone during delayed intensification to determine whether the osteonecrosis risk could be reduced.[
64 ]- The median age at symptom onset was 16 years.
- The cumulative incidence was higher in adolescents and young adults aged 16 to 21 years (20% at 5 years) than in those aged 10 to 15 years (9.9%) or in patients aged 1 to 9 years (1%).
- Operative interventions were needed for management of symptoms and impaired mobility in more than 40% of cases.
- The use of alternate-week dosing of dexamethasone as compared with standard continuous dexamethasone during delayed intensification in CCG-1961 reduced the risk of osteonecrosis. The greatest impact was seen in females aged 16 to 21 years, who showed the highest incidence of osteonecrosis with standard therapy containing continuous dexamethasone; osteonecrosis was reduced with alternate-week dexamethasone postinduction (57.6% to 5.6%).
- In the COG AALL0232 (NCT00075725) high-risk ALL trial, patients were randomly assigned during induction to receive either 14 days of dexamethasone or 28 days of prednisone.[
66 ]- The incidence of osteonecrosis in patients older than 10 years who received dexamethasone was 24.3%, compared with an incidence of 15.9% in those who received prednisone (P = .001).
- For patients aged 10 years and older, there was no difference in outcome between arms by corticosteroid (5-year EFS rate, 73.1% on the dexamethasone arm and 73.9% on the prednisone arm; P = .78).
- However, among all patients in the analysis cohort, patients with osteonecrosis had superior 5-year EFS and OS rates than patients without osteonecrosis (EFS rates, 89.8% vs. 77.8%, P < .0001; OS rates, 95.8% vs. 86.1%, P < .0001).[
67 ] Similar differences were seen in patients older than 10 years. Improved survival was directly attributed to reduced relapse rates.
Treatment options under clinical evaluation for adolescent and young adult patients with ALL
Information about NCI-supported clinical trials can be found on the
The following are examples of national and/or institutional clinical trials that are currently being conducted:
- A041501 (NCT03150693) (Inotuzumab Ozogamicin and Frontline Chemotherapy in Treating Young Adults With Newly Diagnosed B-cell ALL): This is a National Clinical Trials Network trial to further expand on the experience of using a pediatric-inspired chemotherapy backbone in young adults with ALL. Eligibility includes patients aged 18 to 39 years with newly diagnosed CD22-positive ALL. Patients who are in remission after induction will be randomly assigned to receive the pediatric backbone either with or without two courses of inotuzumab ozogamicin (a toxin-conjugated anti-CD22 monoclonal antibody) before starting consolidation therapy.
- COG-AALL1732 (NCT03959085) (A Phase III Randomized Trial of Inotuzumab Ozogamicin for Newly Diagnosed High-Risk B-ALL; Risk-Adapted Postinduction Therapy for High-Risk B-ALL, Mixed Phenotype Acute Leukemia [MPAL], and Disseminated B-Lymphoblastic Lymphoma): This protocol is temporarily closed for patients younger than 25 years at diagnosis who meet any of the following diagnoses: NCI high-risk non-Down syndrome B-ALL, MPAL, and disseminated B-lymphoblastic lymphoma. For patients with B-ALL, the protocol is testing whether the addition of two blocks of inotuzumab ozogamicin to a modified-BFM backbone will improve DFS. For patients with MPAL and disseminated B-lymphoblastic lymphoma, the study aims to determine the EFS associated with treatment using a standard high-risk B-ALL modified-BFM backbone.
Children With Down Syndrome
Approximately 2% to 3% of childhood ALL cases occur in children with Down syndrome.[
Certain genomic abnormalities, such as IKZF1 deletions, CRLF2 aberrations, and JAK variants, are seen more frequently in ALL arising in children with Down syndrome than in those without Down syndrome.[
Patients with Down syndrome have an increased risk of developing toxicities from treatment, including infections, mucositis, and seizures. In some studies, outcomes of children with Down syndrome and ALL have been reported to be inferior,[
Because of the well-established increase in toxicity experienced by patients with Down syndrome, some ALL protocols (such as those of the COG) have de-intensified risk-based treatment for patients with Down syndrome and ALL to minimize exposure to the morbid components of therapy. While this treatment reduction strategy reduces the frequency and severity of toxicities, its impact on antileukemic outcomes is not yet known.
Treatment of children with Down syndrome
Evidence (toxicity and outcome of patients with Down syndrome and ALL):
- A large retrospective study that included 653 patients with Down syndrome and ALL who were treated between 1995 to 2004 reported the following results:[
73 ]- Patients with Down syndrome had lower CR rates (97% vs. 99%, P < .001), higher cumulative incidence of relapse rates (26% vs. 15%, P < .001), and higher treatment-related mortality rates (7.7% vs. 2.3%, P < .001), compared with patients without Down syndrome.
- For patients with Down syndrome and ALL, treatment-related mortality occurred at a higher rate during all phases of therapy, including maintenance. The rates of treatment-related mortality during induction was 2.8% and 4.9% during the remaining treatment phases after CR had been achieved. The most common cause of treatment-related mortality was infection.
- Among the patients with Down syndrome, age younger than 6 years, WBC count of less than 10,000/μL, and the presence of the ETV6::RUNX1 fusion (observed in 8% of patients) were independent predictors of favorable EFS.
- In a report of 2,811 children with ALL enrolled on the COG P9900 classification study, 80 patients (3%) had Down syndrome. Age, sex, WBC count, and risk group were similar between patients with and without Down syndrome, but a lower percentage of patients with Down syndrome had ETV6::RUNX1 (2.5% vs. 24%, P < .001) or trisomies 4 and 10 (7.7% vs. 24%, P < .001).[
70 ]- The 5-year EFS and OS rates were inferior in children with Down syndrome: 69.9% versus 78.1% (P = .078), and 85.8% versus 90.0% (P = .033), respectively.
- However, when children with KMT2A rearrangements, BCR::ABL1, ETV6::RUNX1, and trisomies 4 and 10 were excluded from the analysis, the EFS and OS rates were similar for children with and without Down syndrome (EFS rates, 68.0% [± 9.3%] vs. 70.5% [± 1.9%], P = .817; OS rates, 86.7% [± 6.7%] vs. 85.4% [± 1.5%]; P = .852).
- In the CCG-1991 (NCT00005945) study for children with standard-risk B-ALL (2000–2005), patients (including those with Down syndrome) were randomly assigned to receive an interim maintenance phase consisting of either escalating doses of intravenous methotrexate without leucovorin rescue or standard low-dose oral methotrexate given with vincristine, dexamethasone, and mercaptopurine.[
84 ]- The 10-year EFS rates were 94.4% (± 5.4%) for patients with Down syndrome randomly assigned to receive escalating-dose IV methotrexate (N = 31), versus 81.5% (± 6.6%) for patients who received oral low-dose methotrexate (N = 44).
- The mean total tolerated dose of methotrexate on the escalating IV arm was lower in patients with Down syndrome than patients without Down syndrome.
- The incidence of mucositis was increased in patients with Down syndrome who received escalating-dose methotrexate, compared with patients without Down syndrome, but there was no increase in hepatic toxicity, infections, or treatment-related deaths.
- On DFCI ALL Consortium protocols, children with Down syndrome receive the same risk-stratified therapy as other patients, without any dose reductions or modifications. An analysis of two consecutive trials conducted between 2000 to 2011 found the following:[
83 ]- The 5-year EFS and OS rates of patients with Down syndrome (N = 38) were similar to that of patients without Down syndrome (N = 1,248) (EFS rates, 91% vs. 84%; OS rates, 97% vs. 91%).
- All patients with Down syndrome achieved complete remission, and none of the patients experienced treatment-related mortality.
- Patients with Down syndrome had significantly higher rates of mucositis (52% vs. 12%, P < .001), non-CNS thrombosis (18% vs. 8%; P = .036), and seizure (16% vs. 5%, P = .010). Patients with Down syndrome also had a higher incidence of infections during all therapy phases.
Treatment options under clinical evaluation for children with Down syndrome and ALL
Information about NCI-supported clinical trials can be found on the
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- COG-AALL1731 (NCT03914625) (A Phase III Trial Investigating Blinatumomab in Combination with Chemotherapy in Patients with Newly Diagnosed Standard Risk or Down Syndrome B-ALL and the Treatment of Patients with Localized B-Lymphoblastic Lymphoma): This study is testing whether the addition of blinatumomab to standard chemotherapy will improve DFS. All patients with Down syndrome (including adolescent and young adult patients aged <31 years) are eligible to enroll. NCI standard-risk patients with Down syndrome who meet the definition of standard-risk average will be treated in the same way as patients without Down syndrome who are standard-risk average. These patients are eligible to participate in the blinatumomab randomization. All other patients with Down syndrome, including NCI high-risk patients with Down syndrome, those with unfavorable biology, and those with high day 29 MRD will be considered Down syndrome-high, and will be nonrandomly assigned to receive two cycles of blinatumomab added to a deintensified chemotherapy regimen that omits intensive elements of the augmented BFM treatment backbone. Omitted elements include anthracyclines during induction and cyclophosphamide/cytarabine-based chemotherapy during the second half of delayed intensification.
BCR::ABL1-positive (Philadelphia Chromosome-positive) ALL
BCR::ABL1-positive (Philadelphia chromosome–positive [Ph+]) ALL is seen in about 3% of pediatric ALL cases, increases in adolescence, and is seen in 15% to 25% of adults. In the past, this subtype of ALL has been recognized as extremely difficult to treat, and patients had a poor outcome. In 2000, an international pediatric leukemia group reported a 7-year EFS rate of 25%, with an OS rate of 36%.[
For patients with ALL and BCR::ABL1 gene fusions, MRD detection based on flow cytometry or detection of immunoglobulin/T-cell receptor (IG/TCR) rearrangements by either polymerase chain reaction (PCR) or next-generation sequencing (NGS) provides more reliable prognostication than methods based on quantification of BCR::ABL1 fusion transcripts or DNA.[
Treatment options for patients withBCR::ABL1-positive ALL
Standard therapy for patients with BCR::ABL1 ALL includes the use of a TKI (e.g., imatinib or dasatinib) in combination with cytotoxic chemotherapy, with or without allogeneic HSCT in first CR.
Imatinib mesylate is a selective inhibitor of the BCR::ABL1 protein kinase. Phase I and phase II studies of single-agent imatinib in children and adults with relapsed or refractory BCR::ABL1 ALL have demonstrated relatively high response rates, although these responses tended to be of short duration.[
Clinical trials in adults and children with BCR::ABL1 ALL have demonstrated the feasibility of administering imatinib mesylate in combination with multiagent chemotherapy.[
Dasatinib, a second-generation TKI, has also been studied in the treatment of BCR::ABL1 ALL. Dasatinib has shown significant activity in the CNS, both in a mouse model and a series of patients with CNS-positive leukemia.[
Evidence (TKI):
- The COG-AALL0031 study evaluated whether imatinib mesylate could be incorporated into an intensive chemotherapy regimen for children with BCR::ABL1 ALL. Patients received imatinib mesylate in conjunction with chemotherapy during postinduction therapy. Some children proceeded to allogeneic HSCT after two cycles of consolidation chemotherapy with imatinib mesylate, while other patients received imatinib mesylate in combination with chemotherapy throughout all treatment phases.[
96 ,101 ]- The 5-year DFS rate for the 25 patients who received intensive chemotherapy with continuous dosing of imatinib mesylate was 70% (± 12%). These patients fared better than historical controls who were treated with chemotherapy alone (without imatinib mesylate), and at least as well as the other patients on the trial who underwent allogeneic transplant. The 5-year DFS rate was 66% for patients who underwent sibling-donor transplant (n = 21) and 59% for those who underwent unrelated donor transplant (n = 13).
- Patients with additional cytogenetic abnormalities had worse outcomes (P = .05).
- The COG-AALL0622 (NCT00720109) study tested the use of dasatinib (instead of imatinib) combined with a chemotherapy backbone similar to that used in the COG-AALL0031 trial.[
105 ][Level of evidence B4] On this trial, dasatinib was started on day 15 of induction, while on the AALL0031 trial, imatinib was started postinduction.- Introduction of a TKI during induction on the AALL0631 trial resulted in better early response compared with the AALL0031 trial (no TKI during induction). The end-induction (day 29) CR rate was 98% (n = 59) on the AALL0622 trial, compared with 89% (n = 91) on the AALL0031 trial (P = .01), and rates of low end-induction MRD (<0.01%) were superior on the AALL0622 trial compared with the AALL0031 trial (59% vs. 25%; P < .001).
- Outcomes in the two trials were similar: the 5-year OS rates were 81% and 86%, and the 5-year DFS rates were 68% and 60% for AALL0031 and AALL0622, respectively.
- Excessive toxicity with dasatinib was not observed.
- In a subset analysis that included patients who had diagnostic banked samples available, the IKZF1 deletion was identified in 57% of patients and was associated with inferior EFS and OS.
- The EsPhALL2004 trial tested whether imatinib (administered discontinuously) given in the context of intensive chemotherapy improved outcome for pediatric BCR::ABL1 ALL patients, most of whom (80%) received an allogeneic HSCT in first CR. Patients were classified as either good risk or poor risk on the basis of early response measures and remission status at the end of induction. Good-risk patients (n = 90) were randomly assigned to receive imatinib or no imatinib; poor-risk patients (n = 70) were directly assigned to treatment with imatinib. Interpretation of this study is limited because of the high noncompliance rate with randomized assignment in good-risk patients and early closure before reaching goal accrual because of the publication of the results of the COG AALL0031 trial on which imatinib had been given continuously with chemotherapy.[
102 ]- The overall DFS of patients treated on this trial appeared to be better than historical controls, and when analyzed as-treated (and not by intent-to-treat), good-risk patients who received imatinib had a superior DFS (4-year DFS rate was 75% for patients who received imatinib and 56% for patients who did not receive imatinib).[
106 ]
- The overall DFS of patients treated on this trial appeared to be better than historical controls, and when analyzed as-treated (and not by intent-to-treat), good-risk patients who received imatinib had a superior DFS (4-year DFS rate was 75% for patients who received imatinib and 56% for patients who did not receive imatinib).[
- The subsequent EsPhALL2010 (NCT00287105) trial was a result of an amendment to the 2004 trial, which included earlier initiation of imatinib therapy at day 15 of induction and continuous dosing of imatinib until the end of therapy or 1 year after transplant. A subsequent amendment in the trial also changed the indication for HSCT in first CR to only the poor-risk patients.[
107 ]- Earlier introduction of imatinib (during induction) resulted in an increased rate of CR to 97% at the end of induction (from 78% in the previous trial) and fewer patients being allocated to HSCT (38% on amended trial vs. 81% on initial trial).
- The EFS and OS rates were similar between the amended trial and the initial trial, even though significantly fewer patients received HSCT in first CR on the amended trial.
- The EsPhALL chemotherapy backbone combined with continuous dosing of imatinib was associated with a high rate of toxicity (primarily infections) and treatment-related mortality.
- The CA180-372/COG AALL1122 (NCT01460160) study was a joint COG and European intergroup single-arm, phase II trial that tested the combination of dasatinib and the EsPhALL chemotherapy backbone for patients with newly diagnosed BCR::ABL1–positive ALL. Dasatinib was given starting at day 15 of induction therapy, and only CNS3 patients or those allocated to HSCT received cranial radiation. Nineteen of 109 patients (18%) were considered high risk based on high time point 2 (end-induction IB) MRD or detectable MRD (any level) after three consolidation blocks of chemotherapy. All other patients were considered standard risk (82%). Fifteen patients (14%), all assigned to the high-risk group, underwent HSCT in first CR. All remaining patients were allocated to receive 2 years of chemotherapy with dasatinib.[
89 ]- The 5-year EFS rate was 54.6%, and the 5-year OS rate was 91.5%, indicating a high rate of salvageability after relapse.
- For patients with standard-risk ALL, the 5-year EFS rate was 52.1%, and the OS rate was 82.3%.
- For patients with high-risk ALL, the 5-year EFS rate was 56.8%, and the OS rate was 78.2%.
- The 3-year EFS rate on the AALL1122 trial (65.5%) was statistically noninferior to that of the EsPhALL 2010 trial (59.1%), on which patients received imatinib combined with the same chemotherapy backbone. However, fewer patients on the AALL1122 trial underwent HSCT in first CR, compared with the EsPhALL 2010 trial (14% vs. 38%). Fewer patients on the AALL1122 trial received cranial radiation (15% vs. 100%).
- Frequency of infections were high (grade 3 or higher sepsis, 34%; grade 3 or higher fungal infection, 14%), and the treatment-related mortality rate was 8% (8% in those receiving chemotherapy with dasatinib, 13% in those undergoing HSCT in first CR).
- MRD on this trial was assessed by three methodologies: IG/TCR PCR, flow cytometry, and BCR::ABL1 PCR. Concordance between assays was highest for IG/TCR PCR and flow cytometry, while BCR::ABL1 PCR results were more frequently discordant. The BCR::ABL1 PCR assay more often demonstrated persistent positivity when the other assays yielded nondetectable results. The outcome of patients who continued to have detectable BCR::ABL1 PCR results, but were MRD negative by the other two assays, was similar to that of patients with nondetectable results by all three assays.
- The Chinese Children's Cancer Group ALL-2015 trial randomly assigned 189 patients to receive either dasatinib or imatinib combined with a multiagent regimen based on SJCRH ALL protocols. On this trial, dasatinib was administered at a higher dose (80 mg/m2 instead of 60 mg/m2) and imatinib was administered at a lower dose (300 mg/m2 instead of 340 mg/m2) than they were administered on previous pediatric BCR::ABL1 ALL trials conducted by COG and EsPhALL.[
108 ][Level of evidence A1]- With a median follow-up of 26.4 months, the 4-year EFS and OS rates were 71% and 88.4%, respectively, for patients randomly assigned to receive dasatinib, compared with 48.9% and 69.2%, respectively, for patients randomly assigned to receive imatinib.
- Toxicity was similar between the two treatment arms.
- Caution is needed in interpreting these results because of the short median follow-up time and because the outcomes of patients who received imatinib on this trial were inferior to the outcomes of imatinib-treated patients reported in previous trials.
Treatment options under clinical evaluation forBCR::ABL1ALL
Information about NCI-supported clinical trials can be found on the
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- AALL1631 (NCT03007147) (Imatinib Mesylate and Combination Chemotherapy in Treating Patients with Newly Diagnosed BCR::ABL1 ALL): AALL1631 is an international collaborative protocol conducted by the COG and the European EsPhALL group. Patients with BCR::ABL1 ALL enter the trial at day 15 of induction IA and begin daily imatinib at that time. After the induction IB phase (weeks 10–12), MRD is assessed by immunoglobulin H/T-cell receptor (IgH-TCR) PCR, and patients are classified as standard risk (MRD <0.05%) or high risk (MRD >0.05%). Standard-risk patients are randomly assigned to receive one of the following two cytotoxic chemotherapy backbones:
- The EsPhALL backbone used in previous EsPhALL protocols and COG AALL1122; or
- A less-intensive regimen similar to those typically administered to non-BCR::ABL1 high-risk B-cell ALL patients on COG trials.
Standard-risk patients on both arms will continue to receive imatinib until the completion of all planned chemotherapy (2 years of treatment). The objective of the standard-risk randomization is to determine whether the less-intensive chemotherapy backbone is associated with a similar DFS but lower rates of treatment-related toxicity compared with the standard therapy (EsPhALL chemotherapy backbone).
High-risk patients (approximately 15%–20% of patients) will proceed to HSCT after completion of three consolidation blocks of chemotherapy. Imatinib will be restarted after HSCT and administered from day +56 until day +365 to test the feasibility of post-HSCT administration of this agent and describe the outcome of patients treated in this manner.
Current Clinical Trials
Use our
References:
- Hunger SP, Lu X, Devidas M, et al.: Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children's oncology group. J Clin Oncol 30 (14): 1663-9, 2012.
- Burns MA, Place AE, Stevenson KE, et al.: Identification of prognostic factors in childhood T-cell acute lymphoblastic leukemia: Results from DFCI ALL Consortium Protocols 05-001 and 11-001. Pediatr Blood Cancer 68 (1): e28719, 2021.
- Winter SS, Dunsmore KP, Devidas M, et al.: Improved Survival for Children and Young Adults With T-Lineage Acute Lymphoblastic Leukemia: Results From the Children's Oncology Group AALL0434 Methotrexate Randomization. J Clin Oncol 36 (29): 2926-2934, 2018.
- LeClerc JM, Billett AL, Gelber RD, et al.: Treatment of childhood acute lymphoblastic leukemia: results of Dana-Farber ALL Consortium Protocol 87-01. J Clin Oncol 20 (1): 237-46, 2002.
- Asselin BL, Devidas M, Wang C, et al.: Effectiveness of high-dose methotrexate in T-cell lymphoblastic leukemia and advanced-stage lymphoblastic lymphoma: a randomized study by the Children's Oncology Group (POG 9404). Blood 118 (4): 874-83, 2011.
- Silverman LB, Stevenson KE, O'Brien JE, et al.: Long-term results of Dana-Farber Cancer Institute ALL Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1985-2000). Leukemia 24 (2): 320-34, 2010.
- Asselin BL, Devidas M, Chen L, et al.: Cardioprotection and Safety of Dexrazoxane in Patients Treated for Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia or Advanced-Stage Lymphoblastic Non-Hodgkin Lymphoma: A Report of the Children's Oncology Group Randomized Trial Pediatric Oncology Group 9404. J Clin Oncol 34 (8): 854-62, 2016.
- Chow EJ, Asselin BL, Schwartz CL, et al.: Late Mortality After Dexrazoxane Treatment: A Report From the Children's Oncology Group. J Clin Oncol 33 (24): 2639-45, 2015.
- Seibel NL, Asselin BL, Nachman JB, et al.: Treatment of high risk T-cell acute lymphoblastic leukemia (T-ALL): comparison of recent experience of the Children's Cancer Group (CCG) and Pediatric Oncology Group (POG). [Abstract] Blood 104 (11): A-681, 2004.
- Seibel NL, Steinherz PG, Sather HN, et al.: Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: a report from the Children's Oncology Group. Blood 111 (5): 2548-55, 2008.
- Hastings C, Gaynon PS, Nachman JB, et al.: Increased post-induction intensification improves outcome in children and adolescents with a markedly elevated white blood cell count (≥200 × 10(9) /l) with T cell acute lymphoblastic leukaemia but not B cell disease: a report from the Children's Oncology Group. Br J Haematol 168 (4): 533-46, 2015.
- Matloub Y, Stork L, Asselin B, et al.: Outcome of Children with Standard-Risk T-Lineage Acute Lymphoblastic Leukemia--Comparison among Different Treatment Strategies. Pediatr Blood Cancer 63 (2): 255-61, 2016.
- Berg SL, Blaney SM, Devidas M, et al.: Phase II study of nelarabine (compound 506U78) in children and young adults with refractory T-cell malignancies: a report from the Children's Oncology Group. J Clin Oncol 23 (15): 3376-82, 2005.
- Kurtzberg J, Ernst TJ, Keating MJ, et al.: Phase I study of 506U78 administered on a consecutive 5-day schedule in children and adults with refractory hematologic malignancies. J Clin Oncol 23 (15): 3396-403, 2005.
- Winter SS, Dunsmore KP, Devidas M, et al.: Safe integration of nelarabine into intensive chemotherapy in newly diagnosed T-cell acute lymphoblastic leukemia: Children's Oncology Group Study AALL0434. Pediatr Blood Cancer 62 (7): 1176-83, 2015.
- Dunsmore KP, Devidas M, Linda SB, et al.: Pilot study of nelarabine in combination with intensive chemotherapy in high-risk T-cell acute lymphoblastic leukemia: a report from the Children's Oncology Group. J Clin Oncol 30 (22): 2753-9, 2012.
- Dunsmore KP, Winter SS, Devidas M, et al.: Children's Oncology Group AALL0434: A Phase III Randomized Clinical Trial Testing Nelarabine in Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia. J Clin Oncol 38 (28): 3282-3293, 2020.
- Gossai NP, Devidas M, Chen Z, et al.: Central nervous system status is prognostic in T-cell acute lymphoblastic leukemia: a Children's Oncology Group report. Blood 141 (15): 1802-1811, 2023.
- Teachey DT, Devidas M, Wood BL, et al.: Children's Oncology Group Trial AALL1231: A Phase III Clinical Trial Testing Bortezomib in Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia and Lymphoma. J Clin Oncol 40 (19): 2106-2118, 2022.
- Silverman LB: Acute lymphoblastic leukemia in infancy. Pediatr Blood Cancer 49 (7 Suppl): 1070-3, 2007.
- Hilden JM, Dinndorf PA, Meerbaum SO, et al.: Analysis of prognostic factors of acute lymphoblastic leukemia in infants: report on CCG 1953 from the Children's Oncology Group. Blood 108 (2): 441-51, 2006.
- Pieters R, Schrappe M, De Lorenzo P, et al.: A treatment protocol for infants younger than 1 year with acute lymphoblastic leukaemia (Interfant-99): an observational study and a multicentre randomised trial. Lancet 370 (9583): 240-50, 2007.
- Dreyer ZE, Hilden JM, Jones TL, et al.: Intensified chemotherapy without SCT in infant ALL: results from COG P9407 (Cohort 3). Pediatr Blood Cancer 62 (3): 419-26, 2015.
- Tomizawa D, Miyamura T, Imamura T, et al.: A risk-stratified therapy for infants with acute lymphoblastic leukemia: a report from the JPLSG MLL-10 trial. Blood 136 (16): 1813-1823, 2020.
- van der Linden MH, Valsecchi MG, De Lorenzo P, et al.: Outcome of congenital acute lymphoblastic leukemia treated on the Interfant-99 protocol. Blood 114 (18): 3764-8, 2009.
- Tomizawa D, Koh K, Sato T, et al.: Outcome of risk-based therapy for infant acute lymphoblastic leukemia with or without an MLL gene rearrangement, with emphasis on late effects: a final report of two consecutive studies, MLL96 and MLL98, of the Japan Infant Leukemia Study Group. Leukemia 21 (11): 2258-63, 2007.
- Brown PA, Kairalla JA, Hilden JM, et al.: FLT3 inhibitor lestaurtinib plus chemotherapy for newly diagnosed KMT2A-rearranged infant acute lymphoblastic leukemia: Children's Oncology Group trial AALL0631. Leukemia 35 (5): 1279-1290, 2021.
- Van der Velden VH, Corral L, Valsecchi MG, et al.: Prognostic significance of minimal residual disease in infants with acute lymphoblastic leukemia treated within the Interfant-99 protocol. Leukemia 23 (6): 1073-9, 2009.
- Pieters R, De Lorenzo P, Ancliffe P, et al.: Outcome of Infants Younger Than 1 Year With Acute Lymphoblastic Leukemia Treated With the Interfant-06 Protocol: Results From an International Phase III Randomized Study. J Clin Oncol 37 (25): 2246-2256, 2019.
- Popov A, Tsaur G, Permikin Z, et al.: Incidence and prognostic value of central nervous system involvement in infants with B-cell precursor acute lymphoblastic leukemia treated according to the MLL-Baby protocol. Pediatr Blood Cancer 69 (9): e29860, 2022.
- Ibrahimova A, Winestone LE, Miller TP, et al.: Presentation acuity, induction mortality, and resource utilization in infants with acute leukemia. Pediatr Blood Cancer 68 (7): e28940, 2021.
- Salzer WL, Jones TL, Devidas M, et al.: Decreased induction morbidity and mortality following modification to induction therapy in infants with acute lymphoblastic leukemia enrolled on AALL0631: a report from the Children's Oncology Group. Pediatr Blood Cancer 62 (3): 414-8, 2015.
- Stutterheim J, van der Sluis IM, de Lorenzo P, et al.: Clinical Implications of Minimal Residual Disease Detection in Infants With KMT2A-Rearranged Acute Lymphoblastic Leukemia Treated on the Interfant-06 Protocol. J Clin Oncol 39 (6): 652-662, 2021.
- van der Sluis IM, de Lorenzo P, Kotecha RS, et al.: Blinatumomab Added to Chemotherapy in Infant Lymphoblastic Leukemia. N Engl J Med 388 (17): 1572-1581, 2023.
- Kosaka Y, Koh K, Kinukawa N, et al.: Infant acute lymphoblastic leukemia with MLL gene rearrangements: outcome following intensive chemotherapy and hematopoietic stem cell transplantation. Blood 104 (12): 3527-34, 2004.
- Dreyer ZE, Dinndorf PA, Camitta B, et al.: Analysis of the role of hematopoietic stem-cell transplantation in infants with acute lymphoblastic leukemia in first remission and MLL gene rearrangements: a report from the Children's Oncology Group. J Clin Oncol 29 (2): 214-22, 2011.
- Mann G, Attarbaschi A, Schrappe M, et al.: Improved outcome with hematopoietic stem cell transplantation in a poor prognostic subgroup of infants with mixed-lineage-leukemia (MLL)-rearranged acute lymphoblastic leukemia: results from the Interfant-99 Study. Blood 116 (15): 2644-50, 2010.
- Kato M, Hasegawa D, Koh K, et al.: Allogeneic haematopoietic stem cell transplantation for infant acute lymphoblastic leukaemia with KMT2A (MLL) rearrangements: a retrospective study from the paediatric acute lymphoblastic leukaemia working group of the Japan Society for Haematopoietic Cell Transplantation. Br J Haematol 168 (4): 564-70, 2015.
- Stutterheim J, de Lorenzo P, van der Sluis IM, et al.: Minimal residual disease and outcome characteristics in infant KMT2A-germline acute lymphoblastic leukaemia treated on the Interfant-06 protocol. Eur J Cancer 160: 72-79, 2022.
- Guest EM, Kairalla JA, Hilden JM, et al.: Outstanding outcomes in infants with KMT2A-germline acute lymphoblastic leukemia treated with chemotherapy alone: results of the Children's Oncology Group AALL0631 trial. Haematologica 107 (5): 1205-1208, 2022.
- Nachman J: Clinical characteristics, biologic features and outcome for young adult patients with acute lymphoblastic leukaemia. Br J Haematol 130 (2): 166-73, 2005.
- Pui CH, Pei D, Campana D, et al.: Improved prognosis for older adolescents with acute lymphoblastic leukemia. J Clin Oncol 29 (4): 386-91, 2011.
- Nachman JB, La MK, Hunger SP, et al.: Young adults with acute lymphoblastic leukemia have an excellent outcome with chemotherapy alone and benefit from intensive postinduction treatment: a report from the children's oncology group. J Clin Oncol 27 (31): 5189-94, 2009.
- Pichler H, Reismüller B, Steiner M, et al.: The inferior prognosis of adolescents with acute lymphoblastic leukaemia (ALL) is caused by a higher rate of treatment-related mortality and not an increased relapse rate--a population-based analysis of 25 years of the Austrian ALL-BFM (Berlin-Frankfurt-Münster) Study Group. Br J Haematol 161 (4): 556-65, 2013.
- Burke MJ, Gossai N, Wagner JE, et al.: Survival differences between adolescents/young adults and children with B precursor acute lymphoblastic leukemia after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 19 (1): 138-42, 2013.
- Bhatia S, Landier W, Shangguan M, et al.: Nonadherence to oral mercaptopurine and risk of relapse in Hispanic and non-Hispanic white children with acute lymphoblastic leukemia: a report from the children's oncology group. J Clin Oncol 30 (17): 2094-101, 2012.
- Stock W, La M, Sanford B, et al.: What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? A comparison of Children's Cancer Group and Cancer and Leukemia Group B studies. Blood 112 (5): 1646-54, 2008.
- Ramanujachar R, Richards S, Hann I, et al.: Adolescents with acute lymphoblastic leukaemia: emerging from the shadow of paediatric and adult treatment protocols. Pediatr Blood Cancer 47 (6): 748-56, 2006.
- Barry E, DeAngelo DJ, Neuberg D, et al.: Favorable outcome for adolescents with acute lymphoblastic leukemia treated on Dana-Farber Cancer Institute Acute Lymphoblastic Leukemia Consortium Protocols. J Clin Oncol 25 (7): 813-9, 2007.
- Ramanujachar R, Richards S, Hann I, et al.: Adolescents with acute lymphoblastic leukaemia: outcome on UK national paediatric (ALL97) and adult (UKALLXII/E2993) trials. Pediatr Blood Cancer 48 (3): 254-61, 2007.
- Ram R, Wolach O, Vidal L, et al.: Adolescents and young adults with acute lymphoblastic leukemia have a better outcome when treated with pediatric-inspired regimens: systematic review and meta-analysis. Am J Hematol 87 (5): 472-8, 2012.
- Boissel N, Auclerc MF, Lhéritier V, et al.: Should adolescents with acute lymphoblastic leukemia be treated as old children or young adults? Comparison of the French FRALLE-93 and LALA-94 trials. J Clin Oncol 21 (5): 774-80, 2003.
- Huguet F, Leguay T, Raffoux E, et al.: Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 study. J Clin Oncol 27 (6): 911-8, 2009.
- DeAngelo DJ, Stevenson KE, Dahlberg SE, et al.: Long-term outcome of a pediatric-inspired regimen used for adults aged 18-50 years with newly diagnosed acute lymphoblastic leukemia. Leukemia 29 (3): 526-34, 2015.
- Advani AS, Larsen E, Laumann K, et al.: Comparison of CALGB 10403 (Alliance) and COG AALL0232 toxicity results in young adults with acute lymphoblastic leukemia. Blood Adv 5 (2): 504-512, 2021.
- Ribera JM, Oriol A, Sanz MA, et al.: Comparison of the results of the treatment of adolescents and young adults with standard-risk acute lymphoblastic leukemia with the Programa Español de Tratamiento en Hematología pediatric-based protocol ALL-96. J Clin Oncol 26 (11): 1843-9, 2008.
- Stock W, Luger SM, Advani AS, et al.: A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: results of CALGB 10403. Blood 133 (14): 1548-1559, 2019.
- Wieduwilt MJ, Stock W, Advani A, et al.: Superior survival with pediatric-style chemotherapy compared to myeloablative allogeneic hematopoietic cell transplantation in older adolescents and young adults with Ph-negative acute lymphoblastic leukemia in first complete remission: analysis from CALGB 10403 and the CIBMTR. Leukemia 35 (7): 2076-2085, 2021.
- Gupta S, Pole JD, Baxter NN, et al.: The effect of adopting pediatric protocols in adolescents and young adults with acute lymphoblastic leukemia in pediatric vs adult centers: An IMPACT Cohort study. Cancer Med 8 (5): 2095-2103, 2019.
- Testi AM, Valsecchi MG, Conter V, et al.: Difference in outcome of adolescents with acute lymphoblastic leukemia (ALL) enrolled in pediatric (AIEOP) and adult (GIMEMA) protocols. [Abstract] Blood 104: A-1954, 2004.
- de Bont JM, van der Holt B, Dekker AW, et al.: [Adolescents with acute lymphatic leukaemia achieve significantly better results when treated following Dutch paediatric oncology protocols than with adult protocols]. Ned Tijdschr Geneeskd 149 (8): 400-6, 2005.
- Hallböök H, Gustafsson G, Smedmyr B, et al.: Treatment outcome in young adults and children >10 years of age with acute lymphoblastic leukemia in Sweden: a comparison between a pediatric protocol and an adult protocol. Cancer 107 (7): 1551-61, 2006.
- Hough R, Rowntree C, Goulden N, et al.: Efficacy and toxicity of a paediatric protocol in teenagers and young adults with Philadelphia chromosome negative acute lymphoblastic leukaemia: results from UKALL 2003. Br J Haematol 172 (3): 439-51, 2016.
- Mattano LA, Devidas M, Nachman JB, et al.: Effect of alternate-week versus continuous dexamethasone scheduling on the risk of osteonecrosis in paediatric patients with acute lymphoblastic leukaemia: results from the CCG-1961 randomised cohort trial. Lancet Oncol 13 (9): 906-15, 2012.
- Mogensen SS, Harila-Saari A, Mäkitie O, et al.: Comparing osteonecrosis clinical phenotype, timing, and risk factors in children and young adults treated for acute lymphoblastic leukemia. Pediatr Blood Cancer 65 (10): e27300, 2018.
- Larsen EC, Devidas M, Chen S, et al.: Dexamethasone and High-Dose Methotrexate Improve Outcome for Children and Young Adults With High-Risk B-Acute Lymphoblastic Leukemia: A Report From Children's Oncology Group Study AALL0232. J Clin Oncol 34 (20): 2380-8, 2016.
- Mattano LA, Devidas M, Loh ML, et al.: Development of osteonecrosis and improved survival in B-ALL: results of Children's Oncology Group Trial AALL0232. Leukemia 38 (2): 258-265, 2024.
- Zeller B, Gustafsson G, Forestier E, et al.: Acute leukaemia in children with Down syndrome: a population-based Nordic study. Br J Haematol 128 (6): 797-804, 2005.
- Arico M, Ziino O, Valsecchi MG, et al.: Acute lymphoblastic leukemia and Down syndrome: presenting features and treatment outcome in the experience of the Italian Association of Pediatric Hematology and Oncology (AIEOP). Cancer 113 (3): 515-21, 2008.
- Maloney KW, Carroll WL, Carroll AJ, et al.: Down syndrome childhood acute lymphoblastic leukemia has a unique spectrum of sentinel cytogenetic lesions that influences treatment outcome: a report from the Children's Oncology Group. Blood 116 (7): 1045-50, 2010.
- de Graaf G, Buckley F, Skotko BG: Estimation of the number of people with Down syndrome in the United States. Genet Med 19 (4): 439-447, 2017.
- Chessells JM, Harrison G, Richards SM, et al.: Down's syndrome and acute lymphoblastic leukaemia: clinical features and response to treatment. Arch Dis Child 85 (4): 321-5, 2001.
- Buitenkamp TD, Izraeli S, Zimmermann M, et al.: Acute lymphoblastic leukemia in children with Down syndrome: a retrospective analysis from the Ponte di Legno study group. Blood 123 (1): 70-7, 2014.
- Mullighan CG, Collins-Underwood JR, Phillips LA, et al.: Rearrangement of CRLF2 in B-progenitor- and Down syndrome-associated acute lymphoblastic leukemia. Nat Genet 41 (11): 1243-6, 2009.
- Bercovich D, Ganmore I, Scott LM, et al.: Mutations of JAK2 in acute lymphoblastic leukaemias associated with Down's syndrome. Lancet 372 (9648): 1484-92, 2008.
- Gaikwad A, Rye CL, Devidas M, et al.: Prevalence and clinical correlates of JAK2 mutations in Down syndrome acute lymphoblastic leukaemia. Br J Haematol 144 (6): 930-2, 2009.
- Kearney L, Gonzalez De Castro D, Yeung J, et al.: Specific JAK2 mutation (JAK2R683) and multiple gene deletions in Down syndrome acute lymphoblastic leukemia. Blood 113 (3): 646-8, 2009.
- Buitenkamp TD, Pieters R, Gallimore NE, et al.: Outcome in children with Down's syndrome and acute lymphoblastic leukemia: role of IKZF1 deletions and CRLF2 aberrations. Leukemia 26 (10): 2204-11, 2012.
- Hanada I, Terui K, Ikeda F, et al.: Gene alterations involving the CRLF2-JAK pathway and recurrent gene deletions in Down syndrome-associated acute lymphoblastic leukemia in Japan. Genes Chromosomes Cancer 53 (11): 902-10, 2014.
- Bassal M, La MK, Whitlock JA, et al.: Lymphoblast biology and outcome among children with Down syndrome and ALL treated on CCG-1952. Pediatr Blood Cancer 44 (1): 21-8, 2005.
- Whitlock JA, Sather HN, Gaynon P, et al.: Clinical characteristics and outcome of children with Down syndrome and acute lymphoblastic leukemia: a Children's Cancer Group study. Blood 106 (13): 4043-9, 2005.
- Lundin C, Forestier E, Klarskov Andersen M, et al.: Clinical and genetic features of pediatric acute lymphoblastic leukemia in Down syndrome in the Nordic countries. J Hematol Oncol 7 (1): 32, 2014.
- Athale UH, Puligandla M, Stevenson KE, et al.: Outcome of children and adolescents with Down syndrome treated on Dana-Farber Cancer Institute Acute Lymphoblastic Leukemia Consortium protocols 00-001 and 05-001. Pediatr Blood Cancer 65 (10): e27256, 2018.
- Matloub Y, Rabin KR, Ji L, et al.: Excellent long-term survival of children with Down syndrome and standard-risk ALL: a report from the Children's Oncology Group. Blood Adv 3 (11): 1647-1656, 2019.
- Aricò M, Valsecchi MG, Camitta B, et al.: Outcome of treatment in children with Philadelphia chromosome-positive acute lymphoblastic leukemia. N Engl J Med 342 (14): 998-1006, 2000.
- Aricò M, Schrappe M, Hunger SP, et al.: Clinical outcome of children with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia treated between 1995 and 2005. J Clin Oncol 28 (31): 4755-61, 2010.
- Zuna J, Hovorkova L, Krotka J, et al.: Minimal residual disease in BCR::ABL1-positive acute lymphoblastic leukemia: different significance in typical ALL and in CML-like disease. Leukemia 36 (12): 2793-2801, 2022.
- Short NJ, Jabbour E, Macaron W, et al.: Ultrasensitive NGS MRD assessment in Ph+ ALL: Prognostic impact and correlation with RT-PCR for BCR::ABL1. Am J Hematol 98 (8): 1196-1203, 2023.
- Hunger SP, Tran TH, Saha V, et al.: Dasatinib with intensive chemotherapy in de novo paediatric Philadelphia chromosome-positive acute lymphoblastic leukaemia (CA180-372/COG AALL1122): a single-arm, multicentre, phase 2 trial. Lancet Haematol 10 (7): e510-e520, 2023.
- Hovorkova L, Zaliova M, Venn NC, et al.: Monitoring of childhood ALL using BCR-ABL1 genomic breakpoints identifies a subgroup with CML-like biology. Blood 129 (20): 2771-2781, 2017.
- Duffield AS, Mullighan CG, Borowitz MJ: International Consensus Classification of acute lymphoblastic leukemia/lymphoma. Virchows Arch 482 (1): 11-26, 2023.
- Champagne MA, Capdeville R, Krailo M, et al.: Imatinib mesylate (STI571) for treatment of children with Philadelphia chromosome-positive leukemia: results from a Children's Oncology Group phase 1 study. Blood 104 (9): 2655-60, 2004.
- Ottmann OG, Druker BJ, Sawyers CL, et al.: A phase 2 study of imatinib in patients with relapsed or refractory Philadelphia chromosome-positive acute lymphoid leukemias. Blood 100 (6): 1965-71, 2002.
- Thomas DA, Faderl S, Cortes J, et al.: Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood 103 (12): 4396-407, 2004.
- Yanada M, Takeuchi J, Sugiura I, et al.: High complete remission rate and promising outcome by combination of imatinib and chemotherapy for newly diagnosed BCR-ABL-positive acute lymphoblastic leukemia: a phase II study by the Japan Adult Leukemia Study Group. J Clin Oncol 24 (3): 460-6, 2006.
- Schultz KR, Bowman WP, Aledo A, et al.: Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: a children's oncology group study. J Clin Oncol 27 (31): 5175-81, 2009.
- Burke MJ, Trotz B, Luo X, et al.: Allo-hematopoietic cell transplantation for Ph chromosome-positive ALL: impact of imatinib on relapse and survival. Bone Marrow Transplant 43 (2): 107-13, 2009.
- Lee S, Kim YJ, Min CK, et al.: The effect of first-line imatinib interim therapy on the outcome of allogeneic stem cell transplantation in adults with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood 105 (9): 3449-57, 2005.
- de Labarthe A, Rousselot P, Huguet-Rigal F, et al.: Imatinib combined with induction or consolidation chemotherapy in patients with de novo Philadelphia chromosome-positive acute lymphoblastic leukemia: results of the GRAAPH-2003 study. Blood 109 (4): 1408-13, 2007.
- Rives S, Estella J, Gómez P, et al.: Intermediate dose of imatinib in combination with chemotherapy followed by allogeneic stem cell transplantation improves early outcome in paediatric Philadelphia chromosome-positive acute lymphoblastic leukaemia (ALL): results of the Spanish Cooperative Group SHOP studies ALL-94, ALL-99 and ALL-2005. Br J Haematol 154 (5): 600-11, 2011.
- Schultz KR, Carroll A, Heerema NA, et al.: Long-term follow-up of imatinib in pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia: Children's Oncology Group study AALL0031. Leukemia 28 (7): 1467-71, 2014.
- Biondi A, Schrappe M, De Lorenzo P, et al.: Imatinib after induction for treatment of children and adolescents with Philadelphia-chromosome-positive acute lymphoblastic leukaemia (EsPhALL): a randomised, open-label, intergroup study. Lancet Oncol 13 (9): 936-45, 2012.
- Porkka K, Koskenvesa P, Lundán T, et al.: Dasatinib crosses the blood-brain barrier and is an efficient therapy for central nervous system Philadelphia chromosome-positive leukemia. Blood 112 (4): 1005-12, 2008.
- Zwaan CM, Rizzari C, Mechinaud F, et al.: Dasatinib in children and adolescents with relapsed or refractory leukemia: results of the CA180-018 phase I dose-escalation study of the Innovative Therapies for Children with Cancer Consortium. J Clin Oncol 31 (19): 2460-8, 2013.
- Slayton WB, Schultz KR, Kairalla JA, et al.: Dasatinib Plus Intensive Chemotherapy in Children, Adolescents, and Young Adults With Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia: Results of Children's Oncology Group Trial AALL0622. J Clin Oncol 36 (22): 2306-2314, 2018.
- Biondi A, Cario G, De Lorenzo P, et al.: Long-term follow up of pediatric Philadelphia positive acute lymphoblastic leukemia treated with the EsPhALL2004 study: high white blood cell count at diagnosis is the strongest prognostic factor. Haematologica 104 (1): e13-e16, 2019.
- Biondi A, Gandemer V, De Lorenzo P, et al.: Imatinib treatment of paediatric Philadelphia chromosome-positive acute lymphoblastic leukaemia (EsPhALL2010): a prospective, intergroup, open-label, single-arm clinical trial. Lancet Haematol 5 (12): e641-e652, 2018.
- Shen S, Chen X, Cai J, et al.: Effect of Dasatinib vs Imatinib in the Treatment of Pediatric Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia: A Randomized Clinical Trial. JAMA Oncol 6 (3): 358-366, 2020.
Treatment of Relapsed Childhood ALL
Prognostic Factors After First Relapse of Childhood ALL
The prognosis for a child with acute lymphoblastic leukemia (ALL) whose disease recurs depends on multiple factors.[
The following two important risk factors after first relapse of childhood ALL are key to determining prognosis and treatment approach:
- Site of relapse.
- Time from diagnosis to relapse.
Other prognostic factors include the following:
- Patient characteristics (e.g., age and peripheral blast count at time of relapse).
- Risk group classification at initial diagnosis.
- Response to reinduction therapy.
- Cytogenetics/genomic alterations.
- Immunophenotype.
Site of relapse
Patients who have isolated extramedullary relapse generally fare better than those who have relapse involving the marrow. In some studies, patients with combined marrow/extramedullary relapse had a better prognosis than did those with a marrow only relapse. However, other studies have not confirmed this finding.[
Time from diagnosis to relapse
For patients with relapsed B-ALL, early relapses fare worse than later relapses, and marrow relapses fare worse than isolated extramedullary relapses. For example, survival rates range from less than 20% for patients with marrow relapses occurring within 18 months from diagnosis to higher than 60% for those whose relapses occur more than 36 months from diagnosis.[
For patients with isolated central nervous system (CNS) relapses, the overall survival (OS) rates are 40% to 50% for early relapse (<18 months from diagnosis) and 75% to 80% for those with late relapses (>18 months from diagnosis).[
Patient characteristics
Age 10 years and older at diagnosis and at relapse have been reported as independent predictors of poor outcome.[
For patients with B-ALL who were diagnosed at age 18 years or younger and experienced a late relapse, age was not a significant predictor of subsequent outcome when analyzed by quartiles. However, the outcome for patients aged 18 years and older at time of relapse was significantly inferior to the outcome for patients relapsing at age younger than 18 years (39.5% vs. 68.7%; P = .0001).[
The Berlin-Frankfurt-Münster (BFM) group has also reported that high peripheral blast counts (>10,000/μL) at the time of relapse were associated with inferior outcomes in patients with late marrow relapses.[
Children with Down syndrome and ALL who relapse have generally had inferior outcomes resulting from increased induction deaths, treatment-related mortality, and relapse.
- The BFM group showed that since 2000, improvements in supportive care have led to decreases in treatment-related mortality in children with Down syndrome, but the risk of relapse remains high.[
22 ] - An analysis of data from the Center for International Blood and Marrow Transplant Research (CIBMTR) on 27 patients with Down syndrome and ALL who underwent hematopoietic stem cell transplant (HSCT) between 2000 and 2009 substantiated this finding. They noted that with current transplant practices, hematopoietic recovery, graft-versus-host disease (GVHD), and transplant-related mortality were within the expected range compared with patients without Down syndrome. However, relapse was higher than expected (>50%) and was the primary cause of treatment failure, leading to poor survival (disease-free survival [DFS] rate at 3 years, 24%).[
23 ][Level of evidence C1]
Risk group classification at initial diagnosis
The COG reported that risk group classification at the time of initial diagnosis was prognostically significant after relapse. Patients who met National Cancer Institute (NCI) standard-risk criteria at initial diagnosis fared better after relapse than did NCI high-risk patients.[
Response to reinduction therapy
Patients with marrow relapses who have persistent morphological disease at the end of the first month of reinduction therapy have an extremely poor prognosis, even if they subsequently achieve a second complete remission (CR).[
Cytogenetics/genomic alterations
Changes in variant profiles from diagnosis to relapse have been identified by gene sequencing.[
TP53 alterations (variants and/or copy number alterations) are observed in approximately 10% of patients with ALL at first relapse and have been associated with an increased likelihood of persistent leukemia after initial reinduction and poor event-free survival (EFS) rates.[
IKZF1 deletions have also been reported to be associated with a poor prognosis in patients with B-ALL in first bone marrow relapse.[
RAS pathway variants (KRAS, NRAS, FLT3, and PTPN11) are common at relapse in B-ALL patients, and they were found in approximately 40% of patients at first relapse in one study of 206 children.[
Patients with ETV6::RUNX1-positive ALL appear to have a relatively favorable prognosis at first relapse, consistent with the high percentage of such patients who relapse more than 36 months after diagnosis.[
- In the ALL-REZ BFM 2002 (NCT00114348) study, an EFS rate of 84% (± 7%) was observed for patients with ETV6::RUNX1 ALL with bone marrow relapse.[
38 ] In this study, 94% of patients with ETV6::RUNX1 had a duration of first remission that extended at least 6 months beyond completion of their primary treatment, and on multivariate analysis, time to relapse (and not the presence of ETV6::RUNX1) was an independent predictor of outcome. - Similarly, the 5-year OS rate was 81% for patients with ETV6::RUNX1 enrolled on the French Acute Lymphoblastic Leukaemia Study Group (FRALLE) 93 trial who relapsed at any site more than 36 months after diagnosis. The presence of ETV6::RUNX1 was associated with a favorable survival outcome compared with other late relapsing patients.[
40 ] However, the 3-year OS rate was only 31% for patients with ETV6::RUNX1 who experienced an early relapse (<36 months).
Immunophenotype
Immunophenotype is an important prognostic factor at relapse. Patients with T-ALL who experience a marrow relapse (isolated or combined) at any time during treatment or posttreatment are less likely to achieve a second remission and long-term EFS than are patients with B-ALL.[
Standard Treatment Options for First Bone Marrow Relapse of Childhood ALL
Standard treatment options for first bone marrow relapse include the following:
- Reinduction chemotherapy.
- Postreinduction therapy for patients achieving a second CR.
Reinduction chemotherapy
Initial treatment of relapse consists of reinduction therapy to achieve a second CR. Using either a four-drug reinduction regimen (similar to that administered to newly diagnosed high-risk patients) or an alternative regimen including high-dose methotrexate and high-dose cytarabine, approximately 85% of patients with a marrow relapse achieve a second CR at the end of the first month of treatment.[
Evidence (reinduction chemotherapy):
- A COG study used three blocks of intensive reinduction therapy with an initial four-drug combination that included doxorubicin followed by two intensive consolidation blocks before either HSCT or chemotherapy continuation.[
24 ]- Second remission was achieved after block 1 in 68% of patients with early relapse (<36 months from initial diagnosis) and in 96% of those with later relapse.
- Blocks 2 and 3 reduced MRD in 40 of 56 patients who were MRD positive after block 1.
- A United Kingdom–based randomized trial of ALL patients in first relapse compared reinduction with a four-drug combination using idarubicin versus mitoxantrone.[
42 ][Level of evidence A1]- There was no difference in second CR rates or end-reinduction MRD levels between the two study arms.
- A significant improvement in the OS rate in the mitoxantrone arm was reported (69% vs. 45%; P = .007), which resulted from decreased relapse after transplant.
The potential benefit of mitoxantrone in relapsed ALL regimens requires further investigation.
- Investigators from the ALL-REZ BFM group used a six-drug reinduction approach, which included high-dose methotrexate.[
43 ]- A randomized comparison of 1 g/m2 of methotrexate delivered over 36 hours versus 5 g/m2 of methotrexate delivered over 24 hours with reinduction showed no difference in outcome, but the 36-hour infusion was associated with a higher incidence of mucositis.
- The combination of clofarabine, cyclophosphamide, and etoposide was reported to induce remission in 42% to 56% of patients with refractory or multiply relapsed disease.[
44 ,45 ]; [46 ][Level of evidence B4] - The combination of bortezomib plus vincristine, dexamethasone, pegaspargase, and doxorubicin has been reported to induce complete response (with or without platelet recovery) in 70% to 80% of multiply relapsed patients with B-ALL.[
47 ][Level of evidence C1]; [48 ][Level of evidence C3]- This combination (using prednisone instead of dexamethasone) was tested in B-ALL patients with first relapse occurring less than 36 months from initial diagnosis.[
49 ]- The second CR rate was 68%, which was not significantly different from that observed on a predecessor trial using the same reinduction platform without bortezomib.
- In subset analyses, the addition of bortezomib to the four-drug reinduction platform did not result in significantly better second CR rates for patients with either very early relapses (<18 months from diagnosis) or early relapses (18–36 months from diagnosis) when compared with historical controls.
- This combination (using prednisone instead of dexamethasone) was tested in B-ALL patients with first relapse occurring less than 36 months from initial diagnosis.[
Patients with relapsed T-ALL have much lower rates of achieving second CR with standard reinduction regimens than do patients with B-cell phenotype.[
The proteosome inhibitor bortezomib (in combination with chemotherapy) has also been evaluated in patients with relapsed T-ALL. In a phase II trial conducted by the COG, the combination of bortezomib plus vincristine, prednisone, pegaspargase, and doxorubicin resulted in a second CR rate of 68% in T-ALL patients in first relapse.[
Reinduction failure is a poor prognostic factor, but subsequent attempts to obtain remission can be successful and lead to survival after HSCT, especially if MRD becomes low or nondetectable. For more information about MRD risk stratification, see the Late-relapsing B-ALL section. Approaches have traditionally included the use of drug combinations distinct from the first attempt at treatment. These regimens often contain newer agents under investigation in clinical trials. Although survival is progressively less likely after each attempt, two to four additional attempts are often pursued, with diminishing levels of success measured after each attempt.[
Postreinduction therapy for patients achieving a second complete remission
Early-relapsing B-ALL
For B-ALL patients with an early marrow relapse, allogeneic transplant from an HLA-identical sibling or matched unrelated donor that is performed in second remission has been reported in most studies to result in higher leukemia-free survival (LFS) than a chemotherapy approach.[
After initial reinduction chemotherapy, the use of blinatumomab instead of intensive cytotoxic chemotherapy as pre-HSCT consolidation has been shown to be associated with superior outcomes.[
Evidence (blinatumomab before HSCT in early-relapsing B-ALL):
- In a randomized phase III trial conducted between 2015 and 2019, patients with early-relapsing B-ALL were randomly assigned to receive either one 28-day course of blinatumomab or a cycle of intensive cytotoxic chemotherapy as a third consolidation course before proceeding to HSCT. The study was intended to enroll 202 patients but was terminated early (after 108 patients enrolled) when the superiority of blinatumomab was demonstrated on the basis of a prespecified stopping rule.[
64 ]- The 24-month EFS rate was 66.2% for patients who received blinatumomab, compared with 27.1% for patients who received the intensive chemotherapy block (hazard ratio [HR], 0.33; P < .001).
- The 24-month cumulative incidence of relapse was significantly lower in patients who received blinatumomab than in patients who received intensive chemotherapy (24.9% vs. 70.8%; HR, 0.24).
- For patients with high MRD (≥10-4) at the time randomized treatment began, more patients achieved MRD negativity with blinatumomab treatment than with intensive chemotherapy treatment (93% vs. 24%).
- A COG phase III clinical trial compared two courses of blinatumomab with two blocks of intensive chemotherapy in patients with B-ALL in first relapse. Treatment randomization occurred after a single block of intensive reinduction chemotherapy. Eligible patients included those with early relapse or those with late relapse but MRD levels greater than 0.1% after reinduction chemotherapy. Study enrollment ended early because of loss of clinical equipoise, in favor of blinatumomab. This was determined by the study's data and safety monitoring committee. Key findings from the trial include the following:[
65 ]- The 2-year DFS rate was 54.4% for patients who received blinatumomab versus 39.0% for patients who received chemotherapy (HR for disease progression or mortality, 0.70; 95% confidence interval [CI], 0.47–1.03).
- The 2-year OS rate was 71.3% for patients who received blinatumomab versus 58.4% for patients who received chemotherapy (HR for mortality, 0.62; 95% CI, 0.39–0.98).
- Negative MRD rates were higher for patients after one or two courses of blinatumomab (75% and 66%, respectively) than for patients after one or two courses of intensive chemotherapy (32% and 32%, respectively).
- Adverse event rates for toxicities such as sepsis, mucositis, and febrile neutropenia were much lower for patients who received blinatumomab than for patients who received intensive chemotherapy.
Late-relapsing B-ALL
Previous studies of late marrow relapse in patients with B-ALL showed that a primary chemotherapy approach after achievement of second CR resulted in survival rates of approximately 50%, and it was not clear whether allogeneic transplant was associated with a superior cure rate.[
Evidence (MRD-based risk stratification for late-relapse of B-ALL):
- In BFM studies, patients are considered to be intermediate risk if they have a late isolated marrow relapse or an early or late combined marrow/extramedullary relapse. In the ALL-REZ BFM P95/96 study from this group, end-reinduction MRD (assessed by a polymerase chain reaction–based assay) significantly predicted outcomes of children with intermediate-risk relapsed B-ALL treated with chemotherapy alone in second CR (no HSCT).[
28 ]- Patients with low MRD (<0.1%) had a 10-year EFS rate of 73%, while those with high MRD (≥0.1%) had a 10-year EFS rate of 10%. On multivariate analysis, end-reinduction MRD was the strongest independent prognostic factor.
- In a subsequent BFM study (ALL-REZ BFM 2002 [NCT00114348]), patients with intermediate-risk relapse were allocated to allogeneic HSCT if they had high MRD at the end of the first month of treatment. Those who had low end-reinduction MRD were treated with chemotherapy only (no HSCT).[
70 ]- The EFS rate was 64% for patients with high end-reinduction MRD treated with allogeneic HSCT in second CR, which was significantly better than what had been observed on the previous P95/96 trial, during which such patients received chemotherapy without HSCT. The improvement in EFS was primarily because of a significantly lower risk of relapse in the cohort who underwent HSCT in second CR (cumulative incidence of relapse, 27% on the 2002 trial compared with 59% on the P95/96 trial).
- Patients with late marrow-involved relapses and low end-reinduction MRD, treated with chemotherapy only, had a 5-year EFS rate of 76%, confirming the results seen in the previous P95/96 trial. However, the chemotherapy-only strategy resulted in a significantly worse outcome for patients with early-combined relapses (marrow plus extramedullary site) and low end-reinduction MRD. The 5-year EFS rate was only 37% for these patients. Because of these data, patients with early-combined relapses are now considered high risk on BFM trials.
- For patients with late marrow-involved relapses and high end-reinduction MRD (defined as ≥0.1%), level of MRD was prognostically significant. The 10-year EFS rate was significantly worse for those with MRD levels of ≥1% compared with those with MRD levels of ≥0.1% to <1% (56% vs. 74%, respectively; P = .02). Conversely, for patients with low end-reinduction MRD (<0.1%), there was no significant difference in EFS between those with very low MRD (<0.01%) versus those with MRD levels between 0.01% and <0.1%.[
21 ]
- The United Kingdom ALLR3 trial assigned patients who relapsed more than 6 months after completion of front-line therapy to HSCT if their end-reinduction MRD was ≥0.01% or to chemotherapy for those with MRD of <0.01%.[
17 ]- Of the 228 patients treated, 220 patients achieved CR; 127 patients were allocated to HSCT (high MRD) and 93 were allocated to chemotherapy (low or not evaluable MRD). The 5-year EFS rates were 72% for patients with low MRD versus 56% for patients with high MRD. The 5-year OS rates were 87% for low MRD and 64% for high end-reinduction MRD.
- On the basis of these data, investigators from the United Kingdom recommend HSCT for late-relapsing patients with B-ALL who have MRD of ≥0.01% after reinduction therapy and chemotherapy alone for those with end-reinduction MRD of <0.01%.
- In the COG AALL1331 (NCT02883049) trial, patients with low-risk relapsed ALL were randomly assigned to receive either standard chemotherapy or standard chemotherapy with three blocks of blinatumomab replacing one block of intensive chemotherapy. Low risk was defined as end-reinduction MRD levels of <0.1% in patients with either bone marrow relapse occurring more than 36 months after diagnosis or extramedullary relapse occurring more than 18 months after diagnosis.[
71 ]- For all 255 patients in the protocol, the 4-year DFS rates were 61.2% for patients who received blinatumomab and 49.5% for patients who received standard chemotherapy (P = .0849). The 4-year OS rates were 90.4% for patients who received blinatumomab, compared with 79.6% for patients who received standard chemotherapy (P = .11).
- For patients with bone marrow relapse with or without extramedullary involvement, the 4-year DFS rates were 72.7% for patients who received blinatumomab and 53.7% for those who received standard chemotherapy (P = .015). The 4-year OS rates were 97.1% for patients who received blinatumomab, compared with 84.8% for the chemotherapy-only group (P = .02).
- The outcomes were poor for patients with isolated extramedullary relapse, regardless of treatment arm. Outcomes for these patients were inferior to those of similar patients in previous trials. The 4-year DFS rates were 36.6% for patients who received blinatumomab and 38.8% for those who received chemotherapy only (P = .62). The 4-year OS rates were 76.5% for the blinatumomab group, compared with 68.8% for the chemotherapy-only group (P = .53).
- The COG AALL0433 (NCT00381680) trial included patients with intermediate-risk relapse of B-ALL (defined as late marrow-involved relapse occurring >36 months from initial diagnosis, or very early isolated extramedullary relapse occurring <18 months from initial diagnosis). Patients received three induction blocks, followed either by an intensive chemotherapy backbone (with radiation therapy for patients with CNS or testicular involvement) or allogeneic HSCT (if matched-sibling donors were available). The trial also included a randomized comparison of standard versus intensified vincristine dosing. Of the 271 eligible patients, 257 (95%) achieved second CR. Of these patients, 74 (29% of those in second CR) received HSCT, 47 of whom had matched-sibling donors and 27 of whom had other donors.[
31 ]- For all 271 eligible patients, the 3-year EFS and OS rates were 63.6% and 72.3%, respectively.
- For patients with late marrow-involved relapses (n = 242), the 3-year EFS and OS rates were 66.3% and 74.8%, respectively.
- For patients with late marrow relapses, MRD at the end of the first month of treatment (end-reinduction MRD) was a significant prognostic factor. For patients with end-reinduction MRD less than 0.1%, the 3-year EFS rate was 84.9%, versus 53.7% for those with MRD higher than 0.1%.
- Adjusting for median time to HSCT, patients who received HSCT had an improved 3-year DFS rate (P = .03), but not OS rate (P = .46), compared with patients who received chemotherapy alone.
- The vincristine randomization was stopped early because of excessive toxicity, particularly in older patients.
BCR::ABL1(Philadelphia chromosome–positive [Ph+]) ALL
There is limited information regarding the treatment of patients with relapsed BCR::ABL1 ALL in the era of tyrosine kinase inhibitors (TKIs).
A French multi-institutional study reported on 27 children with relapsed BCR::ABL1 ALL (24 overt, 3 molecular) who had all been initially treated with a regimen that included imatinib.[
- Among patients treated with either an intensive or nonintensive induction regimen, 96% obtained a second CR.
- Approximately one-half of the patients received consolidation therapy, and 78% of the patients proceeded to allogeneic HSCT.
- At the time of relapse, the TKI was changed for 23 of the 26 (88%) patients, 15 of whom (58%) received dasatinib. The 4-year EFS rate was 60.9%, and the OS rate was 76.1%.
T-ALL
For patients with T-ALL who achieve remission after bone marrow relapse, outcomes with postreinduction chemotherapy alone have generally been poor,[
Treatment Options for Second and Subsequent Bone Marrow Relapse
Although there are no studies directly comparing chemotherapy with HSCT for patients in third or subsequent CR, because cure with chemotherapy alone is rare, transplant has generally been considered a reasonable approach for those achieving remission. Long-term survival for ALL patients after a second relapse is particularly poor, in the range of less than 10% to 20%.[
A phase I trial tested the combination of venetoclax (BCL2 inhibitor) and navitoclax (BCL2 and BCL-XL inhibitor) given along with standard chemotherapy (vincristine, dexamethasone, with/without pegaspargase) in adult and pediatric patients with multiply relapsed or refractory B-ALL or T-ALL. The combination was well tolerated in general (the major toxicity was prolonged myelosuppression) and CR was achieved in 60% of patients, 57% of whom had nondetectable MRD.[
For multiply relapsed patients who achieve CR, HSCT has been shown to cure 20% to 35%, with failures occurring because of high rates of relapse and transplant-related mortality.[
Given the poor outcomes for multiply relapsed B-ALL patients who are treated with chemotherapy followed by HSCT, CAR T-cell therapy has come to be used as standard in this population and has resulted in high rates of remission and improved survival (although direct comparative trials are lacking). For more information, see the CAR T-cell therapy section.
Immune therapies such as blinatumomab and inotuzumab have been used in this population and have improved rates of remission, which has then often led to cure when followed by HSCT.[
Hematopoietic Stem Cell Transplant for First and Subsequent Bone Marrow Relapse
Components of the transplant process
An expert panel review of indications for HSCT was published in 2012.[
- Total-body irradiation (TBI)-containing transplant preparative regimens.
- MRD detection just before transplant.
- MRD detection posttransplant.
- Donor type and HLA match.
- Role of GVHD/graft-versus-leukemia (GVL) in ALL and immune modulation after transplant to prevent relapse.
An analysis from the CIBMTR examined pretransplant variables to create a model for predicting LFS posttransplant in pediatric patients (aged <18 years). All patients were first transplant recipients who had myeloablative conditioning, and all stem cells sources were included. For patients with ALL, the predictors associated with lower LFS included age younger than 2 years, second CR or higher, MRD positivity (only in second CR, not in first CR), and presence of morphologically detectable disease at time of transplant. A scale was established to stratify patients on the basis of risk factors to predict survival. The 5-year LFS rate was 68% for the low-risk group, 51% for the intermediate-risk group, and 33% for the high-risk group.[
TBI-containing transplant preparative regimens
For patients proceeding to allogeneic HSCT, TBI appears to be an important component of the conditioning regimen. Several studies have indicated that TBI is associated with superior outcomes in patients with ALL compared with chemotherapy-only preparative regimens.
Evidence (TBI as part of the preparative regimen for ALL):
- Two registry studies and a small randomized trial showed that transplant conditioning regimens that included TBI resulted in higher cure rates than chemotherapy-only preparative regimens.[
55 ,89 ,90 ] - An international study (United States, Europe, and Australia) that combined data sets from prospective trials and single-center data showed that the use of non-TBI regimens was an independent risk factor for poor outcome.[
73 ,80 ,91 ] - An international phase III trial (the FORUM study) conducted between 2013 and 2018 enrolled 417 pediatric patients aged 4 to 21 years with ALL. Patients were randomly assigned to receive either TBI (12 Gy) with etoposide or chemotherapy only (fludarabine, thiotepa, and busulfan or treosulfan) as a preparative regimen. All patients were in morphological CR at the time of transplant and received a 9/10 or 10/10 matched sibling donor (MSD) or unrelated matched donor (URD) transplant. The intent was to enroll 1,000 patients, but the study was closed to enrollment early when a futility early stopping rule was met, indicating that the chemotherapy-only preparative regimen was associated with an inferior outcome.[
92 ]- The 2-year OS rate was 91% for patients who received a TBI-containing regimen, compared with 75% for those who received a chemotherapy-only preparative regimen (P < .001).
- The 2-year EFS rate was also superior in patients who received TBI (86% vs. 58%, P < .001), primarily because of a lower cumulative incidence of relapse in the TBI cohort (2-year cumulative incidence of relapse, 12% for TBI patients vs. 33% for chemotherapy-only patients).
- In subgroup analysis, the TBI regimen was superior regardless of donor type (MSD vs. URD), immunophenotype (B-ALL vs. T-ALL), and whether transplant occurred in first CR or second CR.
- There was no difference in the rates of acute or chronic GVHD or frequency of grades 3 or 4 nonhematologic adverse events between the two randomized treatment arms.
- A substudy of the FORUM trial compared two chemotherapy preparative regimens (busulfan/thiotepa/fludarabine with treosulfan/thiotepa/fludarabine) in 191 children (aged <4 years) who were nonrandomly assigned to non-TBI regimens. The two regimens were also nonrandomly assigned by center/country preference, as some countries did not have access to treosulfan.[
93 ]- The 3-year probabilities of EFS and OS were 0.52 (95% CI, 0.44–0.59) and 0.69 (95% CI, 0.61–0.76), respectively.
- Multivariate analysis showed no difference in EFS and OS according to preparative regimen used, but decreased OS for patients with KMT2A rearrangements and lower EFS in children younger than 1 year at diagnosis.
- A historical comparison was conducted between patients aged 2 to 4 years in this trial and patients aged 2 to 4 years who received TBI regimens in two previous international studies performed by the same group. In the ALL-SCT BFM 2003 trial, 3-year probabilities of EFS and OS were 0.83 (95% CI, 0.66–0.92) and 0.91 (95% CI, 0.76–0.97), respectively. In the ALL-SCT BFM International trial, the 3-year probabilities of EFS and OS were 0.87 (95% CI, 0.57–0.97) and 0.93 (95% CI, 0.61–0.99), respectively. In the FORUM trial, the 3-year probabilities of EFS and OS for children who received either chemotherapeutic conditioning regimen were 0.55 (95% CI, 0.44–0.64) and 0.68 (95% CI, 0.57–0.76), respectively (P = .007 and P = .008 for comparison with the TBI-based trials, respectively).
- The authors concluded that there is strong evidence that TBI-based regimens are superior in children older than 2 years, and this result should be considered as parents and clinicians evaluate the risks and benefits of TBI in children aged 2 to 4 years.
Based on these data, TBI for all but the youngest children (age <2–3 years) remains standard of care in most centers in North America and Europe.[
Fractionated TBI (total dose, 12–14 Gy) is often combined with cyclophosphamide, etoposide, thiotepa, or a combination of these agents. Study findings with these combinations have generally resulted in similar rates of survival,[
A secondary analysis of the COG ASCT0431 (NCT00382109) HSCT trial showed that ALL patients treated with TBI that involved dose modulation of lung fields to less than 8 Gy had a survival advantage on multivariate analysis (hazard ratio [HR], 1.85; P = .04). Transplant-related mortality trended higher for patients who received doses of 8 Gy and higher, but did not reach significance (HR, 1.78; P = .21). Because lower doses were not associated with increased relapse and resulted in improved survival, dose modulation for lung fields to less than 8 Gy was included in the COG AALL1331 (NCT02883049) trial. Results from the AALL1331 study and other studies looking more precisely into pulmonary dose modulation for TBI are needed to clarify and explain this observation.[
MRD detection just before transplant
Remission status at the time of transplant has long been known to be an important predictor of outcome, with patients not in CR at HSCT having very poor survival rates.[
When patients received two to three cycles of chemotherapy in an attempt to achieve an MRD-negative remission, the benefit of further intensive therapy for achieving MRD negativity must be weighed against the potential for significant toxicity. In addition, there is not clear evidence showing that MRD positivity in a patient who has received multiple cycles of therapy is a biological disease marker for poor outcome that cannot be modified, or whether further intervention bringing such patients into an MRD negative remission will overcome this risk factor and improve survival.
- In one report, 13 patients with ALL and high MRD at the time of planned transplant received an additional cycle of chemotherapy in an attempt to lower MRD before proceeding to HSCT. Ten of the 13 patients (77%) remained in CR post-HSCT, with no relapses observed in the eight patients who achieved low MRD after the additional chemotherapy cycle. In comparison, only 6 of 21 high-MRD patients (29%) who proceeded directly to HSCT without receiving additional pre-HSCT chemotherapy remained in CR.[
101 ]
MRD detection posttransplant
The presence of detectable MRD post-HSCT has been associated with an increased risk of subsequent relapse.[
Donor type and HLA match
Survival rates after matched unrelated donor and umbilical cord blood transplant have improved significantly over the past decade and offer an outcome similar to that obtained with matched sibling donor transplants.[
Another CIBMTR study suggested that outcome after one- or two-antigen mismatched cord blood transplants may be equivalent to that for a matched family donor or a matched unrelated donor.[
Role of GVHD/GVL in ALL and immune modulation after transplant to prevent relapse
Most studies of pediatric and young adult patients that address this issue suggest an effect of both acute and chronic GVHD in decreasing relapse.[
- In a COG trial of transplant for children with ALL, grades I to III acute GVHD were associated with lower relapse risk (HR, 0.4; P = .04) and better EFS (multivariate analysis, HR, 0.5; P = .02). Any effect of grade IV acute GVHD in decreasing relapse risk was obscured by a marked increase in transplant-related mortality (HR, 6.4; P = .003), while grades I to III acute GVHD had no statistically detectable effect on transplant-related mortality (HR, 0.6; P = .42).[
135 ] - In a multivariate model, both pretransplant MRD and acute GVHD were independent predictors of relapse, with the lowest risk of relapse observed in patients with both low pretransplant MRD and grades I to III acute GVHD.[
111 ] For patients who did not develop acute GVHD by day 55 post-HSCT, nearly all relapses occurred between days 100 and 400 post-HSCT.
To harness this GVL effect, a number of approaches to prevent relapse after transplant have been studied, including withdrawal of immune suppression or donor lymphocyte infusion and targeted immunotherapies, such as monoclonal antibodies and natural killer cell therapy.[
- One study showed that in 46 patients with increasing recipient chimerism, the 31 patients who underwent immune suppression withdrawal, donor lymphocyte infusion, or both therapies had a 3-year EFS rate of 37% versus 0% in the nonintervention group (P < .001).[
141 ] - Other studies have shown better-than-expected rates of survival of pre-HSCT, MRD-positive patients when tapering of immunosuppression medication has occurred for MRD detected after HSCT.[
142 ] - A large international study showed a marked decrease in relapse in patients who experienced MRD-positive disease after HSCT and developed acute GHVD (HR, 0.29; P < .001), resulting in improved EFS (HR, 2.9; P = .01). Acute GVHD significantly decreased relapse and improved EFS in both MRD-positive and MRD-negative patients. Chronic GVHD was also associated with less relapse in both MRD-positive and MRD-negative patients.[
91 ]
Intrathecal medication after HSCT to prevent relapse
The use of post-HSCT intrathecal chemotherapy chemoprophylaxis is controversial.[
Relapse after allogeneic HSCT for relapsed ALL
For patients with B-ALL who relapse after allogeneic HSCT and can be successfully weaned from immune suppression and have no GVHD, tisagenlecleucel and other 4-1BB CAR T-cell approaches have resulted in EFS rates exceeding 50% at 12 months.[
Reduced-intensity approaches can also cure a percentage of patients when used as a second allogeneic transplant approach, but only if patients achieve a CR confirmed by flow cytometry.[
Whether a second allogeneic transplant is necessary to treat isolated CNS and testicular relapse after HSCT is unknown. A small series has shown survival in selected patients using chemotherapy alone or chemotherapy followed by a second transplant.[
Immunotherapeutic Approaches for Relapsed or Refractory ALL
Immunotherapeutic approaches for the treatment of relapsed or refractory ALL include monoclonal antibody therapy and CAR T-cell therapy.
Monoclonal antibody therapy
The following two immunotherapeutic agents have been studied for the treatment of patients with relapsed or refractory B-ALL:
- Blinatumomab. Blinatumomab is a bispecific monoclonal antibody with one site for CD3 (T cells) and the other site for CD19 (present on most B-ALL cells). Thus, blinatumomab promotes the binding of the patient's own cytotoxic T cells to B lymphoblasts, resulting in the tumor being killed.
In a phase I/II trial of children younger than 18 years with relapsed/refractory B-ALL, 27 of 70 patients (39%) treated at the recommended phase II dose achieved a CR with single-agent blinatumomab; 52% of those achieving CR were MRD negative.[
161 ]In a pooled analysis of five trials that included 166 pediatric and 517 adult patients, those with less than 50% bone marrow blasts at the start of treatment had better responses to blinatumomab. Among pediatric patients, CR rates (including CR with partial hematologic recovery [CRh] and CR with incomplete hematologic recovery [CRi]) were 65.3% for patients with less than 50% baseline bone marrow blast percentage, compared with 38.3% for patients with 50% or more baseline bone marrow blast percentage. Similarly, MRD responses were more frequent in patients with less than 50% baseline bone marrow blast percentage than in those who had 50% or more bone marrow blast percentage (51.4% vs. 25.5%). There was no significant difference in these end points when comparing patients with 5% to <25% blasts and 25% to <50% blasts at the start of blinatumomab treatment.[
162 ] - Inotuzumab. Inotuzumab is an anti-CD22 monoclonal antibody that is conjugated to calicheamicin.
In trials of adult patients with relapsed/refractory B-ALL, CR was achieved in approximately 80% of patients.[
163 ,164 ]There have been two phase II trials of single-agent inotuzumab (both trials used 1.8 mg/m2 total dose in the first course and 1.5 mg/m2 in subsequent courses) used in the treatment of pediatric patients with relapsed (second or greater relapse) or refractory ALL.[
86 ]- In the COG phase II trial (N = 48), the overall response rate (ORR) (CR + CRi) was 58.3% after the first course, and 62.5% after two courses. Of those achieving complete response or complete response with incomplete count recovery, 70% were MRD negative (<0.01%). Overall, inotuzumab was well tolerated, without any significant hepatoxicity or sinusoidal obstructive syndrome (SOS) during treatment. However, of 21 patients who subsequently went on to receive HSCT, 6 (28.6%) developed SOS.[
86 ] - On a phase II trial conducted by the Innovative Therapies for Children With Cancer group (N = 28), the ORR was 81.5% (all responses occurring after the first cycle). Of the patients achieving a response, 81.8% achieved MRD negativity (<0.01%), 59.1% after the first course, and the remaining patients after the second course. SOS was observed in seven (28%) patients, six after subsequent HSCT and one during inotuzumab therapy. There was no correlation between the extent of CD22 expression (mean fluorescence index or percentage of CD22 positive cells) and response. In a post-hoc analysis, combining patients from the phase I and phase II trials, the authors found that having a shorter interval between last dose of inotuzumab and undergoing HSCT was associated with a higher risk of developing SOS.[
165 ]
Expert panel recommendations for the prevention of SOS associated with HSCT after inotuzumab include limiting inotuzumab to two courses, avoiding dual-alkylator HSCT regimens, avoiding hepatotoxic agents, and considering SOS prophylactic agents.[
166 ] - In the COG phase II trial (N = 48), the overall response rate (ORR) (CR + CRi) was 58.3% after the first course, and 62.5% after two courses. Of those achieving complete response or complete response with incomplete count recovery, 70% were MRD negative (<0.01%). Overall, inotuzumab was well tolerated, without any significant hepatoxicity or sinusoidal obstructive syndrome (SOS) during treatment. However, of 21 patients who subsequently went on to receive HSCT, 6 (28.6%) developed SOS.[
- Rituximab or ofatumumab. These monoclonal antibodies target CD20, which is poorly expressed in younger children with ALL, but more common in teenagers and young adults. A number of studies have suggested benefit when adding one of these agents to reinduction chemotherapy in adults,[
167 ,168 ] but very little data have been published in children.
CAR T-cell therapy
Chimeric antigen receptor (CAR) T-cell therapy is a therapeutic strategy for pediatric B-ALL patients with refractory disease or those in second or subsequent relapse. This treatment involves engineering T cells with a CAR that redirects T-cell specificity and function.[
Toxicities associated with CAR T-cell therapy
Treatment with CAR T cells has been associated with cytokine release syndrome, which can be life-threatening.[
Neurotoxicity, including aphasia, altered mental status, and seizures, has also been observed with CAR T-cell therapy, and the symptoms usually resolve spontaneously.[
Other CAR T-cell therapy side effects include coagulopathy, hemophagocytic lymphohistiocytosis (HLH)–like laboratory changes, and cardiac dysfunction. Between 20% and 40% of patients require treatment in the intensive care unit, mostly pressor support, with 10% to 20% of patients requiring intubation and/or dialysis.[
For an extensive discussion of CAR T-cell toxicities and approaches to mitigate these toxicities, see Pediatric Chimeric Antigen Receptor (CAR) T-Cell Therapy.
CD19-targeted CAR T-cell therapy
Several clinical trials of CAR T cells targeting CD19 in relapsed/refractory ALL have been conducted, with encouraging results. Published trials have involved the use of two types of costimulatory molecules, 4-1BB and CD28. CD28-based approaches have led to high rates of remission, but CAR T cells in these trials rarely persist longer than 1 to 2 months, necessitating HSCT for long-term survival.[
Evidence (CD19-targeted CAR T-cell therapy):
- In pilot clinical trials conducted at the Children's Hospital of Philadelphia (CHOP) and the Hospital of the University of Pennsylvania, 30 children and adults (25 of whom were aged 22 years or younger) with multiply relapsed or refractory CD19-positive ALL were given T cells transduced with CD19-directed 4-1BB CAR lentiviral vector.[
170 ][Level of evidence C2]- CR was obtained in 90% of patients, including 15 of 18 patients (83%) who had previously received allogeneic HSCT.
- The 6-month EFS rate was 67%, with most patients showing persistence of the CAR T cells and B-cell aplasia through 6 months.
- All 30 patients experienced some degree of cytokine release syndrome. Eight patients (27%) had severe symptoms requiring vasopressors and/or respiratory support. Cytokine release syndrome was effectively treated with tocilizumab.
- A report of a phase I trial of 45 children and young adults with relapsed/refractory CD19-positive B-ALL who received 4-1BB–based lentiviral vector expanded CAR T cells showed the following:[
174 ]- An overall remission rate of 89% for all patients enrolled using an intent-to-treat analysis.
- Improved long-term persistence of CAR T cells and B-cell aplasia in patients who: (1) received lymphodepleting strategies that contain fludarabine and cyclophosphamide, and (2) started the treatment with a higher percentage of cells expressing CD19, either on blasts or normal B cells.
- A global phase II trial of the anti-CD19 4-1BB vector developed at the CHOP and the University of Pennsylvania led to U.S. Food and Drug Administration approval of tisagenlecleucel for children with multiply relapsed or refractory B-ALL.[
147 ]- Of 92 patients enrolled, 75 were infused with successfully manufactured CAR T cells. Eighty-one percent of infused patients had two measures noting CR within the first 3 months of infusion, and 100% of the remissions were MRD negative.
- The EFS rate of infused patients was 73% at 6 months and 50% at 12 months. The OS rate of infused patients was 90% at 6 months and 70% at 12 months.
- The CIBMTR performed a real-world analysis of 255 patients with ALL that largely duplicated the outcomes shown in the pivotal trials, with an initial CR rate of 85.5%. The 12-month duration of response rate was 69.9%, the EFS rate was 52.4%, and the OS rate was 77.2%.[
179 ]
- Children younger than 3 years were not included in the approval trial for tisagenlecleucel. A subgroup of patients, infants diagnosed at younger than 1 year with KMT2A-rearranged leukemia, often have disease that is highly refractory to intensive chemotherapy approaches. A multicenter retrospective study reported the outcomes of children younger than 3 years (originally diagnosed as high-risk infant ALL) with relapsed or refractory B-ALL who received commercial tisagenlecleucel.[
180 ]- Thirty-eight eligible patients were screened, 35 of whom (92%) were infused with tisagenlecleucel (2 patients did not receive an infusion because CAR T-cell manufacturing failed and 1 patient had disease progression).
- Twenty-nine of 38 patients (76%) had KMT2A rearrangements, and 25 (66%) experienced relapse after previous HSCTs.
- With a median follow-up of 14 months, the 12-month OS rate was 84%, the EFS rate was 69%, and the stringent EFS rate was 41%.
- Feasibility, outcomes, and toxicity were comparable with those of older children.
- A subsequent study from the University of Pennsylvania reported the 3-year updated results of the global phase II ELIANA (NCT02435849) trial that included 79 patients who were treated with tisagenlecleucel.[
181 ][Level of evidence C2]- The overall remission rate was 82% at 3 months.
- With a median follow-up of 38.8 months, the median duration of remission of the 66 patients with a complete response has not been reached. The estimated relapse-free survival (RFS) rate was 58% at 24 months and 52% at 36 months.
- Of the 32 patients in CR who received no additional therapy, the estimated RFS rate was 81% at 24 months and 76% at 36 months.
- A report from the Pediatric Oncology Branch at the NCI described the use of a different CD19-targeted CAR T-cell product with a CD28 costimulatory domain that used a retroviral vector for gene transduction.[
177 ]- This CD19-CAR T-cell product induced complete responses in 70% of patients (14 of 20) (aged 1–30 years) with relapsed/refractory B-ALL.
- Persistence of CAR T cells in this study was 1 to 2 months, with recovery of normal B-cell lymphopoiesis in patients who achieved CR.
- In a subsequent follow-up study of 50 children and young adults who were treated with CD19-CAR T cells, 21 patients achieved an MRD-negative CR and received an allogeneic HSCT. In these 21 patients, the median OS was 70.2 months, and the 5-year EFS rate was 61.9%.[
182 ][Level of evidence C2]
- Another report described a multicenter trial of 25 children and young adults who were treated with anti-CD19, anti-CD28z CAR T cells. Investigators increased the dose of lymphodepleting cyclophosphamide during the trial and analyzed outcomes on the basis of low-dose and high-dose preconditioning, as well as the presence of MRD versus morphological evidence of disease before treatment.[
183 ][Level of evidence A1]- All toxicities were reversible, including 16% of patients with severe cytokine release syndrome and 28% of patients with severe neurotoxicity.
- The overall complete response rate was 75%, and 89% of patients in CR were MRD negative.
- The response rate was superior in the high-dose cyclophosphamide cohort than in the low-dose cohort (94% vs. 38%); the response rate was higher in the MRD cohort than in the morphological disease cohort (93% vs. 50%).
- Of 18 responding patients, 15 underwent consolidative HSCT.
- Improvement in OS only occurred in patients who received the higher dose of cyclophosphamide conditioning.
- In an effort to overcome early loss of functional CD19-targeted CAR T cells, a humanized 4-1BB approach was tested in children and young adults with relapsed or refractory B-ALL or B-lymphoblastic lymphoma. Patients were treated in two cohorts: (1) patients without previous CAR T-cell exposure (CAR-naïve group) and (2) patients with previous CAR exposure who did not respond to more standard CARs, based on mouse-derived antibodies (re-treated group).[
184 ]- CR was achieved in 98% of CAR-naïve patients and 64% of re-treated patients.
- At 6 months, of those who responded, 27% of CAR-naïve patients and 48% of re-treated patients lost CAR persistence.
- For CAR-naive patients, the RFS rate was 84% (95% CI, 72%–97%) at 12 months and 74% (95% CI, 60%–90%) at 24 months.
- For the re-treatment cohort, the RFS rate was 74% (95% CI, 56%–97%) at 12 months and 58% (95% CI, 37%–90%) at 24 months.
- While these results appear favorable, they were not directly compared to currently approved CAR T cells.
CD19-targeted CAR T-cell treatment for CNS and other extramedullary disease
Although there are concerns that the treatment of patients with CNS disease could increase risk of neurotoxicity associated with CAR T-cell therapy, recent studies have shown that patients with CNS disease who undergo CAR T-cell therapy have similar outcomes to those without CNS disease, with no increase in severe immune effector cell associated neurotoxicity syndrome (ICANS).[
Evidence (CAR T-cell therapy for patients with CNS disease):
- Active CNS disease at relapse and/or infusion.
- A single-center report analyzed 195 sequential patients treated with 4-1BB CD19-targeted CAR T cells, 66 of whom had CNS disease (43 with isolated CNS disease, 23 with combined bone marrow/CNS disease).[
185 ]- Overall, there was no difference in remission rates and progression-free survival rates with or without CNS disease.
- Patients with isolated CNS involvement had an RFS rate at 2 years of 66% (95% CI, 52%–82%), compared with 58% (95% CI, 50%–68%) for patients with bone marrow involvement (P = .15).
- Ninety-eight percent of patients were treated before their infusion for their CNS disease and reached CNS1 or CNS2 status at the time of CAR T-cell treatment. Patients with symptomatic/progressive CNS disease were not treated until the disease was controlled and stable.
- A retrospective report examined the outcomes of 184 patients who were treated with tisagenlecleucel at 15 U.S. institutions. In this series, patients were referred for CAR T-cell infusion for marrow-only disease (n = 129), CNS disease (n = 40; either isolated [n = 23] or combined marrow/CNS disease [n = 17]), and non-CNS EMD (n = 15; 6 patients with isolated EMD and 9 with marrow involvement). All but one patient with CNS disease received tisagenlecleucel after one or more relapses (none had previously underwent transplant), whereas 29 of the marrow-only patients (22%) received infusions in first CR, 10 of whom (7.8%) had previously underwent transplant.[
186 ]- In patients referred to receive tisagenlecleucel for CNS disease (n = 40), 31 (78%) were already CNS-negative at the time of infusion, and 88% (35 of 40) were in CR at day 28 after infusion. For patients with non-CNS EMD, 10 of 15 (66%) were in CR at day 28.
- The 12-month RFS rate was 59.4% for patients with marrow-only disease, 59.4% for patients with CNS disease (isolated or combined), and 50% for those with non-CNS EMD (isolated or combined) (P = .92).
- In the CNS-disease cohort, there was no significant difference in 12-month RFS between patients with isolated CNS disease and those with combined CNS and marrow disease (P = .63).
- Grade 3 or 4 neurotoxicity was observed in 3 of 40 patients (7.5%) in the CNS-disease cohort, compared with 9 of 129 patients (7%) with marrow-only disease. None of the patients with active CNS disease at the time of infusion experienced grade 3 or 4 neurotoxicity.
- A second multicenter report reviewed 55 patients with relapses involving the CNS (isolated or combined with bone marrow) who were treated with either a CD28-based institutional CAR (n = 12) or tisagenlecleucel (4-1BB vector, n = 43).[
187 ]- Remission was achieved in 92% of children who received CD28-based CAR T cells and 93% of children who received 4-1BB–based CAR T cells.
- Of 11 children who received CD28-based CAR T cells and achieved a CR, 9 underwent HSCT. Seven of these nine patients survived relapse free.
- None of the 40 patients who were treated with the 4-1BB–based CAR T cells (tisagenlecleucel) and achieved a CR had a planned HSCT. Nineteen of these patients experienced relapses, 12 of whom had CNS relapses.
- Patients who received tisagenlecleucel for an isolated CNS relapse had a high incidence of subsequent CNS relapse (6 of 8 patients).
- A single-center report analyzed 195 sequential patients treated with 4-1BB CD19-targeted CAR T cells, 66 of whom had CNS disease (43 with isolated CNS disease, 23 with combined bone marrow/CNS disease).[
- For non-CNS EMD, available data are limited.
- The report of 195 patients above compared CNS EMD, non-CNS EMD, and bone marrow–only disease. No differences in OS and RFS were noted between the groups.[
185 ] - In contrast, a large retrospective analysis found that active EMD at infusion was independently associated with worse EFS (HR, 1.9; P = .01).[
188 ] - A separate analysis found that in 15 patients with non-CNS EMD, 10 achieved a CR, and 6 of the 10 patients subsequently relapsed. Consequently, only 4 of 15 patients remained event-free during the follow-up period.[
186 ] - A retrospective analysis from the NCI identified limitations in eradication of non-CNS EMD with CAR T cells, despite concurrent marrow responses, highlighting the important role of positron emission tomography scans for monitoring.[
189 ]
- The report of 195 patients above compared CNS EMD, non-CNS EMD, and bone marrow–only disease. No differences in OS and RFS were noted between the groups.[
Risk factors associated with CD19-targeted CAR T-cell failure
Initial trials that used CAR T-cell therapy demonstrated that a very high proportion of patients with relapsed or refractory disease achieved CR, regardless of white blood cell count, cytogenetics,[
- Tumor burden at the time of CAR T-cell infusion.
- A single-center study assessed bone marrow blast percentage immediately before CAR T-cell infusion (after lymphodepleting chemotherapy). High tumor burden (HTB) was defined as 40% blasts or more, and low tumor burden (LTB) was defined as less than 40% blasts.[
172 ]- In the HTB cohort of patients (n = 15), the best ORR was 87%, and the MRD-negative CR rate was 80%.
- In the LTB cohort of patients (n = 55), the best ORR was 100%, and all responders were MRD negative.
- Among HTB patients, the probability of continued remission was 49% (95% CI, 27%–88%) at 12 months and 39% (95% CI, 19%–82%) at 24 months.
- Among LTB patients, the probability of continued remission was 86% (95% CI, 77%–96%) at 12 months and 78% (95% CI, 67%–91%) at 24 months.
- A study of 420 patients assessed the risk factors associated with long-term survival.[
188 ]- On multivariate analysis, a disease burden of 5% or higher, noted by bone marrow flow cytometry before starting lymphodepleting chemotherapy, was associated with a hazard ratio (HR) of 2.52 (95% CI, 1.86–3.41) for inferior EFS, compared with patients who had a lower disease burden.
- In a study of 180 patients who received tisagenlecleucel infusions, univariate and multivariate analyses showed that high-disease burden (>5% bone marrow blasts, CNS3 disease [defined as cerebrospinal fluid specimen with ≥5 white blood cells/μL with lymphoblasts and/or the presence of cranial nerve palsies], or non-CNS extramedullary disease [EMD]) was associated with inferior outcomes.[
191 ]- Patients with a high-disease burden had poorer outcomes (12-month OS rate, 58%; EFS rate, 31%) than patients with a low-disease burden (12-month OS rate, 85%; EFS rate, 70%) and patients with undetectable disease (12-month OS rate, 95%; EFS rate, 72%; P < .0001 for OS and EFS).
- A single-center study assessed bone marrow blast percentage immediately before CAR T-cell infusion (after lymphodepleting chemotherapy). High tumor burden (HTB) was defined as 40% blasts or more, and low tumor burden (LTB) was defined as less than 40% blasts.[
- Previous failure to respond to blinatumomab.
- In a study of 420 pediatric and young adult patients with ALL who were treated with CD19-targeted CAR T cells, the following results were reported:[
188 ]- Patients who had previously failed to respond to blinatumomab before CAR T-cell therapy had lower CR rates after CAR T-cell therapy (20 of 31, 64.5%) compared with previous responders to blinatumomab (39 of 42, 92.9%) or blinatumomab-naïve patients (317 of 339, 93.5%; P < .0001).
- Blinatumomab nonresponders had worse 6-month EFS rates (27.3%; 95% CI, 13.6%–43.0%) than blinatumomab responders (66.9%; 95% CI, 50.6%–78.9%; P < .0001) or blinatumomab-naïve patients (72.6%; 95% CI, 67.5%–77%; P < .0001).
- In a study of 420 pediatric and young adult patients with ALL who were treated with CD19-targeted CAR T cells, the following results were reported:[
- Persistent B-cell aplasia and next-generation sequencing of MRD (NGS-MRD).
- Early studies recognized that B-cell aplasia was a functional measurement of CD19-targeted CAR T-cell persistence, and that loss of B-cell aplasia within the first few months of treatment resulted in high rates of relapse.[
170 ] However, it is an imperfect measurement because CD19-negative relapse can occur despite persistent B-cell aplasia. NGS-MRD techniques rely on clonal rearrangements in the IgH or TCR sequences in lymphoblasts, which typically persist even when CD19 surface expression is lost. - To define the risk of relapse, one study assessed B-cell aplasia and NGS-MRD in bone marrow over time in 143 patients who received tisagenlecleucel.[
192 ]- Multivariate analysis at day 28 showed independent associations of NGS-MRD greater than 0 (HR, 4.87; 95% CI, 2.18–10.8; P < .001) and loss of B-cell aplasia (HR, 3.33; 95% CI, 1.44–7.69; P = .005) with relapse.
- By 3 months, the NGS-MRD HR increased to 12 (95% CI, 2.87–50; P < .001), whereas loss of B-cell aplasia was not independently predictive (HR, 1.27; 95% CI, 0.33–4.79; P = .7).
- Early loss of B-cell aplasia was more predictive of relapse than a later loss of B-cell aplasia.
- Loss of B-cell aplasia in the first 6 months led to an EFS rate lower than 20%; however, patients who lost B-cell aplasia at 1 year had an EFS rate of 75%.
- Notably, NGS-MRD positivity occurring at any time after CAR T-cell therapy led to a very high risk of relapse.
- Early studies recognized that B-cell aplasia was a functional measurement of CD19-targeted CAR T-cell persistence, and that loss of B-cell aplasia within the first few months of treatment resulted in high rates of relapse.[
- CAR T-cell dose.
- A retrospective multicenter study reviewed the doses of CD19-targeted CAR T cells (tisagenlecleucel) given per kilogram by quartiles and assessed patient outcomes.[
193 ]- Patients who received the highest quartile dose had improved OS (HR, 0.22; 95% CI, 0.08–0.61), EFS (HR, 0.24; 95% CI, 0.12–0.49), and RFS (HR, 0.18; 95% CI, 0.07–0.49), compared with those who received the lowest quartile dose.
- Higher doses of CAR T cells were not associated with additional toxicities.
- A potential confounding factor was the significantly higher number of patients with favorable cytogenetics who received the highest quartile dose.
- Further research is warranted to elucidate the relationship between threshold dosing and post-CAR T-cell therapy outcomes for tisagenlecleucel.
- A retrospective multicenter study reviewed the doses of CD19-targeted CAR T cells (tisagenlecleucel) given per kilogram by quartiles and assessed patient outcomes.[
- Timing of relapse after HSCT before treatment with CD19-targeted CAR T cells.
- A retrospective study reported the outcomes of 81 patients with multiply relapsed or primary refractory B-ALL who were treated with tisagenlecleucel. Eighty percent of these patients had previously underwent allogeneic HSCT.[
194 ]- Seventy-one of 81 patients (87.7%) were in remission at day 28 after CAR T-cell therapy.
- At 2 years after CAR T-cell therapy, the EFS rate was 45.3%, and the OS rate was 53.2%.
- Of the patients who previously underwent transplant, time to relapse after HSCT was a significant predictor of outcome when receiving tisagenlecleucel. Patients who had relapsed within 6 months of allogeneic HSCT had an EFS rate of 18.4%, compared with 55.5% for those who relapsed 6 months or more after HSCT.
- A retrospective study reported the outcomes of 81 patients with multiply relapsed or primary refractory B-ALL who were treated with tisagenlecleucel. Eighty percent of these patients had previously underwent allogeneic HSCT.[
Relapse or failure to respond after CD19-targeted CAR T-cell therapy
Retrospective studies have described outcomes and assessed factors associated with survival after relapse in patients who received CD19-targeted CAR T-cell therapy:
- A study looked at 166 relapses occurring after CAR T-cell therapy using either 4-1BB or CD28 costimulatory domains.[
195 ]- Eighty-three patients (50%) were CD19 positive, 68 (41%) were CD19 negative, and 12 (7.2%) had lineage switch relapses.
- The median OS was 11.9 months (95% CI, 9–17 months), with no difference between CD19-positive and CD19-negative relapses.
- Patients with lineage switch relapses had a dismal prognosis, with no long-term survivors.
- CD19-positive relapses were associated with a greater prior treatment burden, shown by a greater number of previous remissions.
- CD19-negative relapses were associated with a greater pre-CAR T-cell infusion disease burden, prior nonresponse to blinatumomab, older age, and a 4-1BB costimulatory domain.
- Lineage switch relapses were associated with the presence of KMT2A rearrangements.
- A second study reviewed 80 patients (27 nonresponders and 53 who relapsed) who received 4-1BB-based CD19-targeted CAR T-cell therapy that failed to produce remissions.[
196 ]- Of the nonresponders, 95% had high disease burden (>5% blasts) before CAR T-cell infusion.
- CD19-negative relapses were associated with a decreased OS rate at 1 year, compared with CD19-positive relapses (30% [95% CI, 14%–66%] vs. 68% [49%–92%]; P = .0068).
- Only treatment followed by HSCT if remission was obtained was associated with long-term survival.
Previous allogeneic donor–derived CD19-targeted CAR T-cell therapy
Certain patients may not be amenable to producing autologous CD19-directed CAR T cells, such as those with very early relapse after HSCT. CAR T-cell therapy using cells produced from the donor of a given patient who is post-HSCT may be an option for these patients. A small group of patients who relapsed after HSCT received allogeneic CD19-directed CAR T cells that were produced from T cells obtained from donors.[
The role of consolidative HSCT after CD19-targeted CAR T-cell therapy for ALL
Studies of second-generation CD19-targeted CAR T-cell approaches have shown that constructs using CD28-based costimulatory molecules result in a relatively short half-life of CAR T cells. This short half-life leads to very high rates of relapse unless HSCT is done soon after recovery from CAR T-cell toxicities. Therefore, treatments with CD28-targeted CAR T cells are considered bridging therapies, and HSCT is generally planned for eligible patients 4 to 8 weeks after the CAR T-cell procedure. Tisagenlecleucel and other CARs with 4-1BB costimulatory molecules have been shown to have significant levels of persistence, leading to long-term remission in 45% to 50% of patients without additional therapy. Up to 80% of relapses occur during the first year after CAR T-cell therapy and there is a window of deep remission. Because of this finding, some study groups have argued for planned HSCT during remission early after CAR T-cell infusion, either in all patients or in patients who have not had a previous HSCT. No randomized trials have addressed this issue, but some studies have addressed this question retrospectively.
Evidence (consolidative HSCT after CAR T-cell therapy):
- CD28 CAR T-cell bridging approach.
- A study examined 50 children and young adults who were treated with CD28-based CD19 CAR T-cell therapy.[
182 ][Level of evidence C2]- Twenty-one patients achieved an MRD-negative CR and underwent an allogeneic HSCT. In these 21 patients, the median OS was 70.2 months, and the 5-year EFS rate was 61.9%.
- A study examined 50 children and young adults who were treated with CD28-based CD19 CAR T-cell therapy.[
- 4-1BB CARs with varying approaches to HSCT.
- A study of 50 patients eligible for HSCT after 4-1BB-based CAR T-cell therapy reported the following results (PLAT-02 [NCT02028455] trial):[
198 ]- Patients who lost CAR T-cell function before 63 days after therapy had improved LFS if they underwent HSCT, compared with those who did not undergo HSCT (P = .01).
- Patients with no prior history of HSCT had an improved LFS with a planned HSCT, with a trend toward significance (P = .09). This benefit was not evident in the 34 patients with a history of prior HSCT (P = .45).
- A study of 50 patients eligible for HSCT after 4-1BB-based CAR T-cell therapy reported the following results (PLAT-02 [NCT02028455] trial):[
- 4-1BB CARs followed by an intent to perform HSCT.
- In a study from China of 52 children who received either CD19- or CD22-targeted CAR T-cell therapy and underwent a planned HSCT at a median of 50 days after CAR T-cell infusion (range, 34–98 days; 48 MRD-negative and 4 MRD-positive patients at time of HSCT), the following results were reported:[
199 ]- The 1-year EFS rate was 73%, and the 1-year OS rate was 87.7%.
- Although the survival rate of patients eligible to proceed to HSCT in remission was high, the analysis did not include patients who had a previous HSCT, patients who did not achieve remission with CAR T-cell infusions, and patients who were not eligible for HSCT after CAR T-cell therapy.
- In a study from China of 52 children who received either CD19- or CD22-targeted CAR T-cell therapy and underwent a planned HSCT at a median of 50 days after CAR T-cell infusion (range, 34–98 days; 48 MRD-negative and 4 MRD-positive patients at time of HSCT), the following results were reported:[
CD22-targeted CAR T-cell therapy
At least 50% of relapses after CD19-targeted CAR T-cell therapy have occurred because of antigen escape, which has been shown to be related to mutations in the CD19 protein that delete the binding sites used by CAR T-cell constructs.[
Evidence (CD22-targeted CAR T-cell therapy):
- Investigators at the NCI reported a phase I/II trial of 58 children and young adults treated with a CD22-targeted CAR T-cell approach.[
204 ][Level of evidence C2]- Of the 55 patients with ALL who were infused, 40 achieved CRs (73%); 88% of those patients achieved MRD-negative remissions.
- Although 86% of patients experienced cytokine release syndrome, 90% experienced grades 1 to 2 cytokine release syndrome.
- Thirty-three percent of patients experienced neurotoxicity, all of which were grades 1 or 2, except in one patient who experienced an intracranial hemorrhage.
- Thirty-eight percent of patients had an HLH/macrophage activation syndrome (MAS)–like syndrome that sometimes required management with anakinra.
- Patients who received CD22-targeted therapy with inotuzumab or CD22 CAR T-cell therapy before this therapy had lower rates of CD22 expression, were less likely to achieve remission, and had shorter duration of remission.
- Thirty of the 40 patients (75%) who achieved CRs relapsed; only patients who proceeded to HSCT had long-term remissions (14 patients proceeded to HSCT, 6 relapsed). The median RFS was 6 months for this group of patients who achieved CRs.
- A Chinese group treated 34 patients who had failed previous CD19-targeted CAR T-cell therapy with CD22-targeted CAR T cells.[
202 ]- CR occurred in 80% of the patients evaluable at day 30 (71% of all patients).
- Seven patients received no further therapy, and three patients remained in remission at 5 to 13 months after therapy.
- Eleven patients went on to HSCT, with a 1-year LFS rate of 72%.
- This study demonstrated that long-term salvage of patients failing CD19-targeted CAR T cells can occur with CD22-targeted CAR T cells plus HSCT.
Multiantigen targeted CAR T-cell therapy
Investigators have tested approaches aimed at targeting multiple ALL antigens to overcome relapse caused by immune escape. Studies have included the following approaches:
- Use of two independently manufactured CAR T cells targeted at different antigens infused simultaneously or sequentially.
- A single manufacturing process of CAR T cells that contain two populations of cells, each targeted at a different antigen.
- A single manufacturing process where one CAR construct has binding domains to multiple antigens.
While none of these approaches are commercially available, studies of this approach are ongoing.
A large study included Chinese patients who received a 1:1 mix of independently manufactured CD19- and CD22-targeted CAR T cells. This treatment produced remission in 99% of patients. Persistent B-cell aplasia at 6 months and HSCT after CAR T cells were each associated with improved survival.[
A second study in China infused CD19-targeted CAR T cells followed by CD22-targeted T cells after achieving remission. This resulted in an 18-month EFS rate of 80%, with HSCT occurring in only 10% of patients.[
Three additional trials examined single manufacturing processes of multitargeted CAR T cells. Treatment with this type of CAR T cells resulted in reasonable rates of remission. However, the duration of CAR T cells was poor, and patient outcomes were no better with this CAR T-cell therapy than outcomes with commercial CD19-targeted CAR T cells.[
Treatment of Isolated Extramedullary Relapse
With improved success in treating children with ALL, the incidence of isolated extramedullary relapse has decreased. The incidence of isolated CNS relapse is less than 5%, and testicular relapse is less than 1% to 2%.[
Isolated CNS relapse
Standard treatment options for childhood ALL that has recurred in the CNS include the following:
- Systemic and intrathecal chemotherapy.
- Cranial or craniospinal radiation.
- HSCT.
- CAR T-cell therapy for isolated CNS disease that is multiply relapsed.
While the prognosis for children with isolated CNS relapse had been quite poor in the past, aggressive systemic and intrathecal therapy followed by cranial or craniospinal radiation has improved the outlook, particularly for patients who did not receive cranial radiation during their first remission.[
Evidence (chemotherapy and radiation therapy):
- In a Pediatric Oncology Group (POG) study using this strategy, children who had not previously received radiation therapy and whose initial remission was 18 months or longer had a 4-year EFS rate of approximately 80%, compared with EFS rates of approximately 45% for children with CNS relapse within 18 months of diagnosis.[
213 ] - In a follow-up POG study, children who had not previously received radiation therapy and who had an initial remission of 18 months or more were treated with intensive systemic and intrathecal chemotherapy for 1 year followed by 18 Gy of cranial radiation only.[
18 ]- The 4-year EFS rate was 78%. Children with an initial remission of less than 18 months also received the same chemotherapy but had craniospinal radiation (24 Gy cranial/15 Gy spinal) as in the first POG study and achieved a 4-year EFS rate of 52%.
- The Children's Oncology Group AALL02P2 (NCT00096135) study enrolled 118 eligible children with B-ALL and late isolated CNS relapse. Patients received intensified systemic therapy, triple intrathecal chemotherapy, and 12 Gy of cranial irradiation delivered at 12 months, with maintenance chemotherapy continuing until 104 weeks postdiagnosis.[
218 ]- The 3-year EFS rate was 64.3% (± 4.5%), and the OS rate was 79.6% (± 3.8%).
- Second relapses that included the CNS occurred in 46.1% of patients (18 of 39).
- Of the 112 patients who completed therapy, 78 received protocol-specified radiation therapy.
- Study enrollment was closed after interim monitoring analysis showed inferior EFS compared with the POG 9412 study, in which patients received 18 Gy of cranial radiation. Thus, the lower dose of cranial radiation was deemed to be inadequate.
A number of case series describing HSCT in the treatment of isolated CNS relapse have been published.[
Evidence (HSCT):
- A retrospective, registry-based study compared the outcome of patients treated with either HLA-matched sibling transplants or chemoradiation therapy as in the POG studies above.[
221 ][Level of evidence C2] This study included transplant of both early (<18 months from diagnosis) and late relapses.- The 8-year probabilities of LFS adjusted for age (58%) and duration of first remission (66%) were similar.
- Because of the relatively good outcome of patients with isolated CNS relapse more than 18 months from diagnosis who were treated with chemoradiation therapy alone (>75%), transplant is generally not recommended by the COG for this group.
- The MRC ALLR3 trial tested intensive induction with mitoxantrone versus idarubicin in relapsed ALL patients, defining a superior outcome when mitoxantrone was used. A subanalysis of 80 patients entering the trial with isolated CNS relapse included 13 patients with very early relapse (defined as <18 months from first diagnosis), 55 patients with early relapse (defined as >18 months from initial diagnosis but within 6 months of being off therapy), and 12 patients with late relapse.[
16 ][Level of evidence B4]- Patients with late relapse did very well with chemotherapy/cranial radiation therapy, with 11 of 12 patients surviving.
- Allogeneic HSCT was recommended for very early and early relapse. Sixty-six patients were alive and relapse free after the planned three induction courses. Fifty-four patients with early and very early isolated CNS relapse were eligible for protocol-recommended HSCT, and 39 patients (72%) received HSCT. Twenty-one percent of these patients relapsed, compared with a relapse rate of 71% in the group that did not receive HSCT.
- Of those eligible for transplant, treatment with mitoxantrone rather than idarubicin during reinduction was associated with a survival advantage (3-year progression-free survival rates, 61% vs. 21%; P = .027). As in the larger trial, the major advantage from the mitoxantrone arm occurred in those who received HSCT. Small patient numbers in the very early group prohibited detailed analysis of this cohort, and rates of failure within the early group treated with chemotherapy/cranial radiation therapy were inferior to other published experiences, calling into question this chemotherapy approach for early isolated CNS relapse patients.
Evidence (CAR T-cell therapy for isolated CNS disease that is multiply relapsed):
CAR T cells have been shown to penetrate the CNS and lead to high rates of remission in patients with CNS disease with or without marrow involvement. A small number of studies have addressed the relationship of CNS involvement with CAR T-cell therapy outcomes.
- A single-center study reported on 195 sequential patients treated with 4-1BB CD19-targeted CAR T cells, 66 of whom had CNS disease (43 with isolated CNS disease, 23 with combined BM/CNS disease).[
185 ]- Overall, there was no difference in remission rates and progression-free survival in patients with or without CNS disease.
- Of the patients with CNS disease, 79% were second or greater relapse.
- Patients with isolated CNS involvement had an RFS rate at 2 years of 66% (95% CI, 52%–82%), compared with 58% (95% CI, 50%–68%) for patients with bone marrow involvement (P = .15).
Isolated testicular relapse
The results of treatment of isolated testicular relapse depend on the timing of the relapse. The 3-year EFS rate of boys with overt testicular relapse during therapy is approximately 40%; it is approximately 85% for boys with late testicular relapse.[
Standard treatment options in North America for childhood ALL that has recurred in the testes include the following:
- Chemotherapy.
- Radiation therapy.
Standard approaches for treating isolated testicular relapse in North America include local radiation therapy along with intensive chemotherapy. In some European clinical trial groups, orchiectomy of the involved testicle is performed instead of radiation. Biopsy of the other testicle is performed at the time of relapse to determine if additional local control (surgical removal or radiation) is to be performed. A study that looked at testicular biopsy at the end of frontline therapy failed to demonstrate a survival benefit for patients with early detection of occult disease.[
There are limited clinical data concerning outcome without the use of radiation therapy or orchiectomy. Treatment protocols that have tested this approach have incorporated intensified dosing of chemotherapy agents (e.g., high-dose methotrexate) that may be able to achieve antileukemic levels in the testes.
Evidence (treatment of testicular relapse):
- The COG AALL02P2 (NCT00096135) trial tested whether radiation therapy could be eliminated for patients with late isolated testicular relapse (occurring more than 18 months from diagnosis).[
224 ] On this trial, testicular size was reassessed after the initial month of reinduction chemotherapy, which included high-dose methotrexate. If the testicle remained enlarged, biopsy was performed, and if positive, patients were to be treated with local radiation therapy. Those with testes that normalized in size or who had negative biopsies were to be treated without radiation therapy. Postinduction chemotherapy for all patients (whether or not they were irradiated) included multiple courses of high-dose methotrexate.[225 ]- Of 40 patients enrolled, 26 had persistent testicular enlargement after reinduction. Testicular biopsy was positive in 12 of these 26 patients, 11 of whom received testicular radiation therapy; all other patients on the trial were treated without radiation.
- Participants who received testicular radiation therapy achieved a 5-year EFS rate of 73% versus 61% for those who did not receive radiation (P = .6); the 5-year OS rate was 73% for those who received testicular radiation versus 71% (P = .9) for those who did not receive testicular radiation.
- Thus, for patients with isolated testicular relapse achieving a favorable response after initial induction (documented by size reduction and/or biopsy), omission of testicular radiation therapy may be a feasible option.
- Dutch investigators treated five boys with a late testicular relapse with high-dose methotrexate during induction (12 g/m2) and at regular intervals during the remainder of therapy (6 g/m2) without testicular radiation.[
224 ]- All five boys were long-term survivors.
- In a small series of boys who had an isolated testicular relapse after an HSCT for a prior systemic relapse of ALL, five of seven boys had extended EFS without a second HSCT.[
160 ][Level of evidence C1]
Treatment Options Under Clinical Evaluation for Relapsed Childhood ALL
Trials for ALL in first relapse
Information about NCI-supported clinical trials can be found on the
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- COG-AALL1821 (NCT04546399) (A Study to Compare Blinatumomab Alone to Blinatumomab With Nivolumab in Patients Diagnosed With First Relapse B-ALL): This protocol is testing the incorporation of immunotherapeutic therapies into the treatment of patients with B-ALL who are in first marrow relapse. Patients are stratified into three groups on the basis of age at time of study entry and timing of relapse from initial diagnosis.
- Group 1 patients are either: (1) older than 18 years (regardless of timing of relapse) or (2) younger than 18 years with relapse occurring less than 24 months from initial diagnosis. These patients are randomly assigned to receive either blinatumomab alone or blinatumomab in combination with nivolumab (a checkpoint inhibitor), as reinduction for one or two cycles, followed by allogeneic HSCT.
All patients who do not qualify for group 1 (i.e., those who are younger than 18 years with relapse occurring more than 24 months from initial diagnosis) receive a four-drug reinduction (vincristine, dexamethasone, doxorubicin, and pegaspargase) and are then classified as either group 2 or group 3.
- Group 2 patients are younger than 18 years with either: (1) relapse occurring between 24 months and 36 months from diagnosis, regardless of end-reinduction MRD or (2) relapse occurring more than 36 months from diagnosis with MRD greater than 0.1% after four-drug reinduction. These patients are randomly assigned to receive either blinatumomab alone or blinatumomab in combination with nivolumab, as postinduction consolidation, before proceeding to allogeneic HSCT.
- Group 3 patients are younger than 18 years with relapse occurring more than 36 months from diagnosis and MRD less than 0.1% after four-drug reinduction and are not allocated to HSCT. These patients are randomly assigned to receive standard consolidation chemotherapy alternating with either three cycles of blinatumomab monotherapy or blinatumomab in combination with nivolumab, followed by maintenance therapy.
Isolated extramedullary patients are not eligible for this trial.
Trials for ALL in second or subsequent relapse or refractory ALL
Multiple clinical trials investigating new agents and new combinations of agents are available for children with second or subsequent relapsed or refractory ALL and should be considered. These trials are testing targeted treatments specific for ALL, including monoclonal antibody–based therapies and drugs that inhibit signal transduction pathways required for leukemia cell growth and survival. Multiple clinical trials investigating new agents, new combinations of agents, and immunotherapeutic approaches are available. For more information, see the
Current Clinical Trials
Use our
References:
- Reismüller B, Attarbaschi A, Peters C, et al.: Long-term outcome of initially homogenously treated and relapsed childhood acute lymphoblastic leukaemia in Austria--a population-based report of the Austrian Berlin-Frankfurt-Münster (BFM) Study Group. Br J Haematol 144 (4): 559-70, 2009.
- Uderzo C, Conter V, Dini G, et al.: Treatment of childhood acute lymphoblastic leukemia after the first relapse: curative strategies. Haematologica 86 (1): 1-7, 2001.
- Chessells JM, Veys P, Kempski H, et al.: Long-term follow-up of relapsed childhood acute lymphoblastic leukaemia. Br J Haematol 123 (3): 396-405, 2003.
- Rivera GK, Zhou Y, Hancock ML, et al.: Bone marrow recurrence after initial intensive treatment for childhood acute lymphoblastic leukemia. Cancer 103 (2): 368-76, 2005.
- Einsiedel HG, von Stackelberg A, Hartmann R, et al.: Long-term outcome in children with relapsed ALL by risk-stratified salvage therapy: results of trial acute lymphoblastic leukemia-relapse study of the Berlin-Frankfurt-Münster Group 87. J Clin Oncol 23 (31): 7942-50, 2005.
- Schroeder H, Garwicz S, Kristinsson J, et al.: Outcome after first relapse in children with acute lymphoblastic leukemia: a population-based study of 315 patients from the Nordic Society of Pediatric Hematology and Oncology (NOPHO). Med Pediatr Oncol 25 (5): 372-8, 1995.
- Wheeler K, Richards S, Bailey C, et al.: Comparison of bone marrow transplant and chemotherapy for relapsed childhood acute lymphoblastic leukaemia: the MRC UKALL X experience. Medical Research Council Working Party on Childhood Leukaemia. Br J Haematol 101 (1): 94-103, 1998.
- Buchanan GR, Rivera GK, Pollock BH, et al.: Alternating drug pairs with or without periodic reinduction in children with acute lymphoblastic leukemia in second bone marrow remission: a Pediatric Oncology Group Study. Cancer 88 (5): 1166-74, 2000.
- Rivera GK, Hudson MM, Liu Q, et al.: Effectiveness of intensified rotational combination chemotherapy for late hematologic relapse of childhood acute lymphoblastic leukemia. Blood 88 (3): 831-7, 1996.
- Bührer C, Hartmann R, Fengler R, et al.: Peripheral blast counts at diagnosis of late isolated bone marrow relapse of childhood acute lymphoblastic leukemia predict response to salvage chemotherapy and outcome. Berlin-Frankfurt-Münster Relapse Study Group. J Clin Oncol 14 (10): 2812-7, 1996.
- Roy A, Cargill A, Love S, et al.: Outcome after first relapse in childhood acute lymphoblastic leukaemia - lessons from the United Kingdom R2 trial. Br J Haematol 130 (1): 67-75, 2005.
- Rizzari C, Valsecchi MG, Aricò M, et al.: Outcome of very late relapse in children with acute lymphoblastic leukemia. Haematologica 89 (4): 427-34, 2004.
- Nguyen K, Devidas M, Cheng SC, et al.: Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children's Oncology Group study. Leukemia 22 (12): 2142-50, 2008.
- Locatelli F, Schrappe M, Bernardo ME, et al.: How I treat relapsed childhood acute lymphoblastic leukemia. Blood 120 (14): 2807-16, 2012.
- Malempati S, Gaynon PS, Sather H, et al.: Outcome after relapse among children with standard-risk acute lymphoblastic leukemia: Children's Oncology Group study CCG-1952. J Clin Oncol 25 (36): 5800-7, 2007.
- Masurekar AN, Parker CA, Shanyinde M, et al.: Outcome of central nervous system relapses in childhood acute lymphoblastic leukaemia--prospective open cohort analyses of the ALLR3 trial. PLoS One 9 (10): e108107, 2014.
- Parker C, Krishnan S, Hamadeh L, et al.: Outcomes of patients with childhood B-cell precursor acute lymphoblastic leukaemia with late bone marrow relapses: long-term follow-up of the ALLR3 open-label randomised trial. Lancet Haematol 6 (4): e204-e216, 2019.
- Barredo JC, Devidas M, Lauer SJ, et al.: Isolated CNS relapse of acute lymphoblastic leukemia treated with intensive systemic chemotherapy and delayed CNS radiation: a pediatric oncology group study. J Clin Oncol 24 (19): 3142-9, 2006.
- Rubnitz JE, Hijiya N, Zhou Y, et al.: Lack of benefit of early detection of relapse after completion of therapy for acute lymphoblastic leukemia. Pediatr Blood Cancer 44 (2): 138-41, 2005.
- Freyer DR, Devidas M, La M, et al.: Postrelapse survival in childhood acute lymphoblastic leukemia is independent of initial treatment intensity: a report from the Children's Oncology Group. Blood 117 (11): 3010-5, 2011.
- Eckert C, Groeneveld-Krentz S, Kirschner-Schwabe R, et al.: Improving Stratification for Children With Late Bone Marrow B-Cell Acute Lymphoblastic Leukemia Relapses With Refined Response Classification and Integration of Genetics. J Clin Oncol 37 (36): 3493-3506, 2019.
- Meyr F, Escherich G, Mann G, et al.: Outcomes of treatment for relapsed acute lymphoblastic leukaemia in children with Down syndrome. Br J Haematol 162 (1): 98-106, 2013.
- Hitzler JK, He W, Doyle J, et al.: Outcome of transplantation for acute lymphoblastic leukemia in children with Down syndrome. Pediatr Blood Cancer 61 (6): 1126-8, 2014.
- Raetz EA, Borowitz MJ, Devidas M, et al.: Reinduction platform for children with first marrow relapse in acute lymphoblastic lymphoma. J Clin Oncol 26 (24): 3971-8, 2008.
- von Stackelberg A, Völzke E, Kühl JS, et al.: Outcome of children and adolescents with relapsed acute lymphoblastic leukaemia and non-response to salvage protocol therapy: a retrospective analysis of the ALL-REZ BFM Study Group. Eur J Cancer 47 (1): 90-7, 2011.
- Coustan-Smith E, Gajjar A, Hijiya N, et al.: Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia after first relapse. Leukemia 18 (3): 499-504, 2004.
- Sramkova L, Muzikova K, Fronkova E, et al.: Detectable minimal residual disease before allogeneic hematopoietic stem cell transplantation predicts extremely poor prognosis in children with acute lymphoblastic leukemia. Pediatr Blood Cancer 48 (1): 93-100, 2007.
- Eckert C, von Stackelberg A, Seeger K, et al.: Minimal residual disease after induction is the strongest predictor of prognosis in intermediate risk relapsed acute lymphoblastic leukaemia - long-term results of trial ALL-REZ BFM P95/96. Eur J Cancer 49 (6): 1346-55, 2013.
- Eckert C, Parker C, Moorman AV, et al.: Risk factors and outcomes in children with high-risk B-cell precursor and T-cell relapsed acute lymphoblastic leukaemia: combined analysis of ALLR3 and ALL-REZ BFM 2002 clinical trials. Eur J Cancer 151: 175-189, 2021.
- Paganin M, Zecca M, Fabbri G, et al.: Minimal residual disease is an important predictive factor of outcome in children with relapsed 'high-risk' acute lymphoblastic leukemia. Leukemia 22 (12): 2193-200, 2008.
- Lew G, Chen Y, Lu X, et al.: Outcomes after late bone marrow and very early central nervous system relapse of childhood B-acute lymphoblastic leukemia: a report from the Children's Oncology Group phase III study AALL0433. Haematologica 106 (1): 46-55, 2021.
- Ma X, Edmonson M, Yergeau D, et al.: Rise and fall of subclones from diagnosis to relapse in pediatric B-acute lymphoblastic leukaemia. Nat Commun 6: 6604, 2015.
- Mullighan CG, Zhang J, Kasper LH, et al.: CREBBP mutations in relapsed acute lymphoblastic leukaemia. Nature 471 (7337): 235-9, 2011.
- Brady SW, Roberts KG, Gu Z, et al.: The genomic landscape of pediatric acute lymphoblastic leukemia. Nat Genet 54 (9): 1376-1389, 2022.
- Meyer JA, Wang J, Hogan LE, et al.: Relapse-specific mutations in NT5C2 in childhood acute lymphoblastic leukemia. Nat Genet 45 (3): 290-4, 2013.
- Tzoneva G, Perez-Garcia A, Carpenter Z, et al.: Activating mutations in the NT5C2 nucleotidase gene drive chemotherapy resistance in relapsed ALL. Nat Med 19 (3): 368-71, 2013.
- Hof J, Krentz S, van Schewick C, et al.: Mutations and deletions of the TP53 gene predict nonresponse to treatment and poor outcome in first relapse of childhood acute lymphoblastic leukemia. J Clin Oncol 29 (23): 3185-93, 2011.
- Krentz S, Hof J, Mendioroz A, et al.: Prognostic value of genetic alterations in children with first bone marrow relapse of childhood B-cell precursor acute lymphoblastic leukemia. Leukemia 27 (2): 295-304, 2013.
- Irving J, Matheson E, Minto L, et al.: Ras pathway mutations are prevalent in relapsed childhood acute lymphoblastic leukemia and confer sensitivity to MEK inhibition. Blood 124 (23): 3420-30, 2014.
- Gandemer V, Chevret S, Petit A, et al.: Excellent prognosis of late relapses of ETV6/RUNX1-positive childhood acute lymphoblastic leukemia: lessons from the FRALLE 93 protocol. Haematologica 97 (11): 1743-50, 2012.
- Tallen G, Ratei R, Mann G, et al.: Long-term outcome in children with relapsed acute lymphoblastic leukemia after time-point and site-of-relapse stratification and intensified short-course multidrug chemotherapy: results of trial ALL-REZ BFM 90. J Clin Oncol 28 (14): 2339-47, 2010.
- Parker C, Waters R, Leighton C, et al.: Effect of mitoxantrone on outcome of children with first relapse of acute lymphoblastic leukaemia (ALL R3): an open-label randomised trial. Lancet 376 (9757): 2009-17, 2010.
- von Stackelberg A, Hartmann R, Bührer C, et al.: High-dose compared with intermediate-dose methotrexate in children with a first relapse of acute lymphoblastic leukemia. Blood 111 (5): 2573-80, 2008.
- Locatelli F, Testi AM, Bernardo ME, et al.: Clofarabine, cyclophosphamide and etoposide as single-course re-induction therapy for children with refractory/multiple relapsed acute lymphoblastic leukaemia. Br J Haematol 147 (3): 371-8, 2009.
- Miano M, Pistorio A, Putti MC, et al.: Clofarabine, cyclophosphamide and etoposide for the treatment of relapsed or resistant acute leukemia in pediatric patients. Leuk Lymphoma 53 (9): 1693-8, 2012.
- Hijiya N, Thomson B, Isakoff MS, et al.: Phase 2 trial of clofarabine in combination with etoposide and cyclophosphamide in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. Blood 118 (23): 6043-9, 2011.
- Bertaina A, Vinti L, Strocchio L, et al.: The combination of bortezomib with chemotherapy to treat relapsed/refractory acute lymphoblastic leukaemia of childhood. Br J Haematol 176 (4): 629-636, 2017.
- Messinger YH, Gaynon PS, Sposto R, et al.: Bortezomib with chemotherapy is highly active in advanced B-precursor acute lymphoblastic leukemia: Therapeutic Advances in Childhood Leukemia & Lymphoma (TACL) Study. Blood 120 (2): 285-90, 2012.
- Horton TM, Whitlock JA, Lu X, et al.: Bortezomib reinduction chemotherapy in high-risk ALL in first relapse: a report from the Children's Oncology Group. Br J Haematol 186 (2): 274-285, 2019.
- Berg SL, Blaney SM, Devidas M, et al.: Phase II study of nelarabine (compound 506U78) in children and young adults with refractory T-cell malignancies: a report from the Children's Oncology Group. J Clin Oncol 23 (15): 3376-82, 2005.
- Zwaan CM, Kowalczyk J, Schmitt C, et al.: Safety and efficacy of nelarabine in children and young adults with relapsed or refractory T-lineage acute lymphoblastic leukaemia or T-lineage lymphoblastic lymphoma: results of a phase 4 study. Br J Haematol 179 (2): 284-293, 2017.
- Commander LA, Seif AE, Insogna IG, et al.: Salvage therapy with nelarabine, etoposide, and cyclophosphamide in relapsed/refractory paediatric T-cell lymphoblastic leukaemia and lymphoma. Br J Haematol 150 (3): 345-51, 2010.
- Whitlock JA, Malvar J, Dalla-Pozza L, et al.: Nelarabine, etoposide, and cyclophosphamide in relapsed pediatric T-acute lymphoblastic leukemia and T-lymphoblastic lymphoma (study T2008-002 NECTAR). Pediatr Blood Cancer 69 (11): e29901, 2022.
- Sun W, Malvar J, Sposto R, et al.: Outcome of children with multiply relapsed B-cell acute lymphoblastic leukemia: a therapeutic advances in childhood leukemia & lymphoma study. Leukemia 32 (11): 2316-2325, 2018.
- Eapen M, Raetz E, Zhang MJ, et al.: Outcomes after HLA-matched sibling transplantation or chemotherapy in children with B-precursor acute lymphoblastic leukemia in a second remission: a collaborative study of the Children's Oncology Group and the Center for International Blood and Marrow Transplant Research. Blood 107 (12): 4961-7, 2006.
- Barrett AJ, Horowitz MM, Pollock BH, et al.: Bone marrow transplants from HLA-identical siblings as compared with chemotherapy for children with acute lymphoblastic leukemia in a second remission. N Engl J Med 331 (19): 1253-8, 1994.
- Uderzo C, Valsecchi MG, Bacigalupo A, et al.: Treatment of childhood acute lymphoblastic leukemia in second remission with allogeneic bone marrow transplantation and chemotherapy: ten-year experience of the Italian Bone Marrow Transplantation Group and the Italian Pediatric Hematology Oncology Association. J Clin Oncol 13 (2): 352-8, 1995.
- Harrison G, Richards S, Lawson S, et al.: Comparison of allogeneic transplant versus chemotherapy for relapsed childhood acute lymphoblastic leukaemia in the MRC UKALL R1 trial. MRC Childhood Leukaemia Working Party. Ann Oncol 11 (8): 999-1006, 2000.
- Bunin N, Carston M, Wall D, et al.: Unrelated marrow transplantation for children with acute lymphoblastic leukemia in second remission. Blood 99 (9): 3151-7, 2002.
- Borgmann A, von Stackelberg A, Hartmann R, et al.: Unrelated donor stem cell transplantation compared with chemotherapy for children with acute lymphoblastic leukemia in a second remission: a matched-pair analysis. Blood 101 (10): 3835-9, 2003.
- Saarinen-Pihkala UM, Heilmann C, Winiarski J, et al.: Pathways through relapses and deaths of children with acute lymphoblastic leukemia: role of allogeneic stem-cell transplantation in Nordic data. J Clin Oncol 24 (36): 5750-62, 2006.
- Thomson B, Park JR, Felgenhauer J, et al.: Toxicity and efficacy of intensive chemotherapy for children with acute lymphoblastic leukemia (ALL) after first bone marrow or extramedullary relapse. Pediatr Blood Cancer 43 (5): 571-9, 2004.
- Hahn T, Wall D, Camitta B, et al.: The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of acute lymphoblastic leukemia in children: an evidence-based review. Biol Blood Marrow Transplant 11 (11): 823-61, 2005.
- Locatelli F, Zugmaier G, Rizzari C, et al.: Effect of Blinatumomab vs Chemotherapy on Event-Free Survival Among Children With High-risk First-Relapse B-Cell Acute Lymphoblastic Leukemia: A Randomized Clinical Trial. JAMA 325 (9): 843-854, 2021.
- Brown PA, Ji L, Xu X, et al.: Effect of Postreinduction Therapy Consolidation With Blinatumomab vs Chemotherapy on Disease-Free Survival in Children, Adolescents, and Young Adults With First Relapse of B-Cell Acute Lymphoblastic Leukemia: A Randomized Clinical Trial. JAMA 325 (9): 833-842, 2021.
- Borgmann A, Baumgarten E, Schmid H, et al.: Allogeneic bone marrow transplantation for a subset of children with acute lymphoblastic leukemia in third remission: a conceivable alternative? Bone Marrow Transplant 20 (11): 939-44, 1997.
- Schroeder H, Gustafsson G, Saarinen-Pihkala UM, et al.: Allogeneic bone marrow transplantation in second remission of childhood acute lymphoblastic leukemia: a population-based case control study from the Nordic countries. Bone Marrow Transplant 23 (6): 555-60, 1999.
- van den Berg H, de Groot-Kruseman HA, Damen-Korbijn CM, et al.: Outcome after first relapse in children with acute lymphoblastic leukemia: a report based on the Dutch Childhood Oncology Group (DCOG) relapse all 98 protocol. Pediatr Blood Cancer 57 (2): 210-6, 2011.
- Beck JC, Cao Q, Trotz B, et al.: Allogeneic hematopoietic cell transplantation outcomes for children with B-precursor acute lymphoblastic leukemia and early or late BM relapse. Bone Marrow Transplant 46 (7): 950-5, 2011.
- Eckert C, Henze G, Seeger K, et al.: Use of allogeneic hematopoietic stem-cell transplantation based on minimal residual disease response improves outcomes for children with relapsed acute lymphoblastic leukemia in the intermediate-risk group. J Clin Oncol 31 (21): 2736-42, 2013.
- Hogan LE, Brown PA, Ji L, et al.: Children's Oncology Group AALL1331: Phase III Trial of Blinatumomab in Children, Adolescents, and Young Adults With Low-Risk B-Cell ALL in First Relapse. J Clin Oncol 41 (25): 4118-4129, 2023.
- Aubert L, Petit A, Bertrand Y, et al.: Therapeutic approach and outcome of children with Philadelphia chromosome-positive acute lymphoblastic leukemia at first relapse in the era of tyrosine kinase inhibitors: An SFCE retrospective study. Pediatr Blood Cancer 69 (2): e29441, 2022.
- Burke MJ, Verneris MR, Le Rademacher J, et al.: Transplant Outcomes for Children with T Cell Acute Lymphoblastic Leukemia in Second Remission: A Report from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant 21 (12): 2154-9, 2015.
- Gaynon PS: Childhood acute lymphoblastic leukaemia and relapse. Br J Haematol 131 (5): 579-87, 2005.
- Jeha S, Goto H, Baruchel A, et al.: Patient-Level Meta-analysis of Clofarabine in Acute Lymphoblastic Leukemia. Adv Ther 40 (12): 5447-5463, 2023.
- Pullarkat VA, Lacayo NJ, Jabbour E, et al.: Venetoclax and Navitoclax in Combination with Chemotherapy in Patients with Relapsed or Refractory Acute Lymphoblastic Leukemia and Lymphoblastic Lymphoma. Cancer Discov 11 (6): 1440-1453, 2021.
- Woolfrey AE, Anasetti C, Storer B, et al.: Factors associated with outcome after unrelated marrow transplantation for treatment of acute lymphoblastic leukemia in children. Blood 99 (6): 2002-8, 2002.
- Afify Z, Hunt L, Green A, et al.: Factors affecting the outcome of stem cell transplantation from unrelated donors for childhood acute lymphoblastic leukemia in third remission. Bone Marrow Transplant 35 (11): 1041-7, 2005.
- Gassas A, Ishaqi MK, Afzal S, et al.: Outcome of haematopoietic stem cell transplantation for paediatric acute lymphoblastic leukaemia in third complete remission: a vital role for graft-versus-host-disease/ graft-versus-leukaemia effect in survival. Br J Haematol 140 (1): 86-9, 2008.
- Nemecek ER, Ellis K, He W, et al.: Outcome of myeloablative conditioning and unrelated donor hematopoietic cell transplantation for childhood acute lymphoblastic leukemia in third remission. Biol Blood Marrow Transplant 17 (12): 1833-40, 2011.
- Kato M, Horikoshi Y, Okamoto Y, et al.: Second allogeneic hematopoietic SCT for relapsed ALL in children. Bone Marrow Transplant 47 (10): 1307-11, 2012.
- Bhojwani D, Sposto R, Shah NN, et al.: Inotuzumab ozogamicin in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. Leukemia 33 (4): 884-892, 2019.
- Pulsipher MA: Are CAR T cells better than antibody or HCT therapy in B-ALL? Hematology Am Soc Hematol Educ Program 2018 (1): 16-24, 2018.
- Contreras CF, Higham CS, Behnert A, et al.: Clinical utilization of blinatumomab and inotuzumab immunotherapy in children with relapsed or refractory B-acute lymphoblastic leukemia. Pediatr Blood Cancer 68 (1): e28718, 2021.
- Locatelli F, Maschan A, Boissel N, et al.: Pediatric patients with acute lymphoblastic leukemia treated with blinatumomab in a real-world setting: Results from the NEUF study. Pediatr Blood Cancer 69 (4): e29562, 2022.
- O'Brien MM, Ji L, Shah NN, et al.: Phase II Trial of Inotuzumab Ozogamicin in Children and Adolescents With Relapsed or Refractory B-Cell Acute Lymphoblastic Leukemia: Children's Oncology Group Protocol AALL1621. J Clin Oncol 40 (9): 956-967, 2022.
- Oliansky DM, Camitta B, Gaynon P, et al.: Role of cytotoxic therapy with hematopoietic stem cell transplantation in the treatment of pediatric acute lymphoblastic leukemia: update of the 2005 evidence-based review. Biol Blood Marrow Transplant 18 (4): 505-22, 2012.
- Qayed M, Ahn KW, Kitko CL, et al.: A validated pediatric disease risk index for allogeneic hematopoietic cell transplantation. Blood 137 (7): 983-993, 2021.
- Davies SM, Ramsay NK, Klein JP, et al.: Comparison of preparative regimens in transplants for children with acute lymphoblastic leukemia. J Clin Oncol 18 (2): 340-7, 2000.
- Bunin N, Aplenc R, Kamani N, et al.: Randomized trial of busulfan vs total body irradiation containing conditioning regimens for children with acute lymphoblastic leukemia: a Pediatric Blood and Marrow Transplant Consortium study. Bone Marrow Transplant 32 (6): 543-8, 2003.
- Bader P, Salzmann-Manrique E, Balduzzi A, et al.: More precisely defining risk peri-HCT in pediatric ALL: pre- vs post-MRD measures, serial positivity, and risk modeling. Blood Adv 3 (21): 3393-3405, 2019.
- Peters C, Dalle JH, Locatelli F, et al.: Total Body Irradiation or Chemotherapy Conditioning in Childhood ALL: A Multinational, Randomized, Noninferiority Phase III Study. J Clin Oncol 39 (4): 295-307, 2021.
- Bader P, Pötschger U, Dalle JH, et al.: Low rate of nonrelapse mortality in under-4-year-olds with ALL given chemotherapeutic conditioning for HSCT: a phase 3 FORUM study. Blood Adv 8 (2): 416-428, 2024.
- Gassas A, Sung L, Saunders EF, et al.: Comparative outcome of hematopoietic stem cell transplantation for pediatric acute lymphoblastic leukemia following cyclophosphamide and total body irradiation or VP16 and total body irradiation conditioning regimens. Bone Marrow Transplant 38 (11): 739-43, 2006.
- Tracey J, Zhang MJ, Thiel E, et al.: Transplantation conditioning regimens and outcomes after allogeneic hematopoietic cell transplantation in children and adolescents with acute lymphoblastic leukemia. Biol Blood Marrow Transplant 19 (2): 255-9, 2013.
- Bakr M, Rasheed W, Mohamed SY, et al.: Allogeneic hematopoietic stem cell transplantation in adolescent and adult patients with high-risk T cell acute lymphoblastic leukemia. Biol Blood Marrow Transplant 18 (12): 1897-904, 2012.
- Marks DI, Forman SJ, Blume KG, et al.: A comparison of cyclophosphamide and total body irradiation with etoposide and total body irradiation as conditioning regimens for patients undergoing sibling allografting for acute lymphoblastic leukemia in first or second complete remission. Biol Blood Marrow Transplant 12 (4): 438-53, 2006.
- Esiashvili N, Lu X, Ulin K, et al.: Higher Reported Lung Dose Received During Total Body Irradiation for Allogeneic Hematopoietic Stem Cell Transplantation in Children With Acute Lymphoblastic Leukemia Is Associated With Inferior Survival: A Report from the Children's Oncology Group. Int J Radiat Oncol Biol Phys 104 (3): 513-521, 2019.
- Duval M, Klein JP, He W, et al.: Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol 28 (23): 3730-8, 2010.
- Shen Z, Gu X, Mao W, et al.: Influence of pre-transplant minimal residual disease on prognosis after Allo-SCT for patients with acute lymphoblastic leukemia: systematic review and meta-analysis. BMC Cancer 18 (1): 755, 2018.
- Balduzzi A, Di Maio L, Silvestri D, et al.: Minimal residual disease before and after transplantation for childhood acute lymphoblastic leukaemia: is there any room for intervention? Br J Haematol 164 (3): 396-408, 2014.
- Goulden N, Bader P, Van Der Velden V, et al.: Minimal residual disease prior to stem cell transplant for childhood acute lymphoblastic leukaemia. Br J Haematol 122 (1): 24-9, 2003.
- Bader P, Kreyenberg H, Henze GH, et al.: Prognostic value of minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia: the ALL-REZ BFM Study Group. J Clin Oncol 27 (3): 377-84, 2009.
- Leung W, Pui CH, Coustan-Smith E, et al.: Detectable minimal residual disease before hematopoietic cell transplantation is prognostic but does not preclude cure for children with very-high-risk leukemia. Blood 120 (2): 468-72, 2012.
- Ruggeri A, Michel G, Dalle JH, et al.: Impact of pretransplant minimal residual disease after cord blood transplantation for childhood acute lymphoblastic leukemia in remission: an Eurocord, PDWP-EBMT analysis. Leukemia 26 (12): 2455-61, 2012.
- Bachanova V, Burke MJ, Yohe S, et al.: Unrelated cord blood transplantation in adult and pediatric acute lymphoblastic leukemia: effect of minimal residual disease on relapse and survival. Biol Blood Marrow Transplant 18 (6): 963-8, 2012.
- Sutton R, Shaw PJ, Venn NC, et al.: Persistent MRD before and after allogeneic BMT predicts relapse in children with acute lymphoblastic leukaemia. Br J Haematol 168 (3): 395-404, 2015.
- Sanchez-Garcia J, Serrano J, Serrano-Lopez J, et al.: Quantification of minimal residual disease levels by flow cytometry at time of transplant predicts outcome after myeloablative allogeneic transplantation in ALL. Bone Marrow Transplant 48 (3): 396-402, 2013.
- Ifversen M, Turkiewicz D, Marquart HV, et al.: Low burden of minimal residual disease prior to transplantation in children with very high risk acute lymphoblastic leukaemia: The NOPHO ALL2008 experience. Br J Haematol 184 (6): 982-993, 2019.
- Bader P, Kreyenberg H, von Stackelberg A, et al.: Monitoring of minimal residual disease after allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia allows for the identification of impending relapse: results of the ALL-BFM-SCT 2003 trial. J Clin Oncol 33 (11): 1275-84, 2015.
- Pulsipher MA, Langholz B, Wall DA, et al.: Risk factors and timing of relapse after allogeneic transplantation in pediatric ALL: for whom and when should interventions be tested? Bone Marrow Transplant 50 (9): 1173-9, 2015.
- Pulsipher MA, Carlson C, Langholz B, et al.: IgH-V(D)J NGS-MRD measurement pre- and early post-allotransplant defines very low- and very high-risk ALL patients. Blood 125 (22): 3501-8, 2015.
- Liu J, Wang Y, Xu LP, et al.: Monitoring mixed lineage leukemia expression may help identify patients with mixed lineage leukemia--rearranged acute leukemia who are at high risk of relapse after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 20 (7): 929-36, 2014.
- Locatelli F, Zecca M, Messina C, et al.: Improvement over time in outcome for children with acute lymphoblastic leukemia in second remission given hematopoietic stem cell transplantation from unrelated donors. Leukemia 16 (11): 2228-37, 2002.
- Saarinen-Pihkala UM, Gustafsson G, Ringdén O, et al.: No disadvantage in outcome of using matched unrelated donors as compared with matched sibling donors for bone marrow transplantation in children with acute lymphoblastic leukemia in second remission. J Clin Oncol 19 (14): 3406-14, 2001.
- Muñoz A, Diaz-Heredia C, Diaz MA, et al.: Allogeneic hemopoietic stem cell transplantation for childhood acute lymphoblastic leukemia in second complete remission-similar outcomes after matched related and unrelated donor transplant: a study of the Spanish Working Party for Blood and Marrow Transplantation in Children (Getmon). Pediatr Hematol Oncol 25 (4): 245-59, 2008.
- Jacobsohn DA, Hewlett B, Ranalli M, et al.: Outcomes of unrelated cord blood transplants and allogeneic-related hematopoietic stem cell transplants in children with high-risk acute lymphocytic leukemia. Bone Marrow Transplant 34 (10): 901-7, 2004.
- Kurtzberg J, Prasad VK, Carter SL, et al.: Results of the Cord Blood Transplantation Study (COBLT): clinical outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with hematologic malignancies. Blood 112 (10): 4318-27, 2008.
- Peters C, Schrappe M, von Stackelberg A, et al.: Stem-cell transplantation in children with acute lymphoblastic leukemia: A prospective international multicenter trial comparing sibling donors with matched unrelated donors-The ALL-SCT-BFM-2003 trial. J Clin Oncol 33 (11): 1265-74, 2015.
- Smith AR, Baker KS, Defor TE, et al.: Hematopoietic cell transplantation for children with acute lymphoblastic leukemia in second complete remission: similar outcomes in recipients of unrelated marrow and umbilical cord blood versus marrow from HLA matched sibling donors. Biol Blood Marrow Transplant 15 (9): 1086-93, 2009.
- Zhang MJ, Davies SM, Camitta BM, et al.: Comparison of outcomes after HLA-matched sibling and unrelated donor transplantation for children with high-risk acute lymphoblastic leukemia. Biol Blood Marrow Transplant 18 (8): 1204-10, 2012.
- Gassas A, Sung L, Saunders EF, et al.: Graft-versus-leukemia effect in hematopoietic stem cell transplantation for pediatric acute lymphoblastic leukemia: significantly lower relapse rate in unrelated transplantations. Bone Marrow Transplant 40 (10): 951-5, 2007.
- Harvey J, Green A, Cornish J, et al.: Improved survival in matched unrelated donor transplant for childhood ALL since the introduction of high-resolution matching at HLA class I and II. Bone Marrow Transplant 47 (10): 1294-300, 2012.
- Majhail NS, Chitphakdithai P, Logan B, et al.: Significant improvement in survival after unrelated donor hematopoietic cell transplantation in the recent era. Biol Blood Marrow Transplant 21 (1): 142-50, 2015.
- MacMillan ML, Davies SM, Nelson GO, et al.: Twenty years of unrelated donor bone marrow transplantation for pediatric acute leukemia facilitated by the National Marrow Donor Program. Biol Blood Marrow Transplant 14 (9 Suppl): 16-22, 2008.
- Davies SM, Wang D, Wang T, et al.: Recent decrease in acute graft-versus-host disease in children with leukemia receiving unrelated donor bone marrow transplants. Biol Blood Marrow Transplant 15 (3): 360-6, 2009.
- Eapen M, Rubinstein P, Zhang MJ, et al.: Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet 369 (9577): 1947-54, 2007.
- Klingebiel T, Handgretinger R, Lang P, et al.: Haploidentical transplantation for acute lymphoblastic leukemia in childhood. Blood Rev 18 (3): 181-92, 2004.
- Ruggeri A, Galimard JE, Paina O, et al.: Outcomes of Unmanipulated Haploidentical Transplantation Using Post-Transplant Cyclophosphamide (PT-Cy) in Pediatric Patients With Acute Lymphoblastic Leukemia. Transplant Cell Ther 27 (5): 424.e1-424.e9, 2021.
- Locatelli F, Merli P, Pagliara D, et al.: Outcome of children with acute leukemia given HLA-haploidentical HSCT after αβ T-cell and B-cell depletion. Blood 130 (5): 677-685, 2017.
- Bertaina A, Zecca M, Buldini B, et al.: Unrelated donor vs HLA-haploidentical α/β T-cell- and B-cell-depleted HSCT in children with acute leukemia. Blood 132 (24): 2594-2607, 2018.
- Pulsipher MA, Ahn KW, Bunin NJ, et al.: KIR-favorable TCR-αβ/CD19-depleted haploidentical HCT in children with ALL/AML/MDS: primary analysis of the PTCTC ONC1401 trial. Blood 140 (24): 2556-2572, 2022.
- Gustafsson Jernberg A, Remberger M, Ringdén O, et al.: Graft-versus-leukaemia effect in children: chronic GVHD has a significant impact on relapse and survival. Bone Marrow Transplant 31 (3): 175-81, 2003.
- Dini G, Zecca M, Balduzzi A, et al.: No difference in outcome between children and adolescents transplanted for acute lymphoblastic leukemia in second remission. Blood 118 (25): 6683-90, 2011.
- Pulsipher MA, Langholz B, Wall DA, et al.: The addition of sirolimus to tacrolimus/methotrexate GVHD prophylaxis in children with ALL: a phase 3 Children's Oncology Group/Pediatric Blood and Marrow Transplant Consortium trial. Blood 123 (13): 2017-25, 2014.
- Yeshurun M, Weisdorf D, Rowe JM, et al.: The impact of the graft-versus-leukemia effect on survival in acute lymphoblastic leukemia. Blood Adv 3 (4): 670-680, 2019.
- Pulsipher MA, Bader P, Klingebiel T, et al.: Allogeneic transplantation for pediatric acute lymphoblastic leukemia: the emerging role of peritransplantation minimal residual disease/chimerism monitoring and novel chemotherapeutic, molecular, and immune approaches aimed at preventing relapse. Biol Blood Marrow Transplant 15 (1 Suppl): 62-71, 2008.
- Lankester AC, Bierings MB, van Wering ER, et al.: Preemptive alloimmune intervention in high-risk pediatric acute lymphoblastic leukemia patients guided by minimal residual disease level before stem cell transplantation. Leukemia 24 (8): 1462-9, 2010.
- Horn B, Soni S, Khan S, et al.: Feasibility study of preemptive withdrawal of immunosuppression based on chimerism testing in children undergoing myeloablative allogeneic transplantation for hematologic malignancies. Bone Marrow Transplant 43 (6): 469-76, 2009.
- Pochon C, Oger E, Michel G, et al.: Follow-up of post-transplant minimal residual disease and chimerism in childhood lymphoblastic leukaemia: 90 d to react. Br J Haematol 169 (2): 249-61, 2015.
- Bader P, Kreyenberg H, Hoelle W, et al.: Increasing mixed chimerism is an important prognostic factor for unfavorable outcome in children with acute lymphoblastic leukemia after allogeneic stem-cell transplantation: possible role for pre-emptive immunotherapy? J Clin Oncol 22 (9): 1696-705, 2004.
- Gandemer V, Pochon C, Oger E, et al.: Clinical value of pre-transplant minimal residual disease in childhood lymphoblastic leukaemia: the results of the French minimal residual disease-guided protocol. Br J Haematol 165 (3): 392-401, 2014.
- Rubin J, Vettenranta K, Vettenranta J, et al.: Use of intrathecal chemoprophylaxis in children after SCT and the risk of central nervous system relapse. Bone Marrow Transplant 46 (3): 372-8, 2011.
- Thompson CB, Sanders JE, Flournoy N, et al.: The risks of central nervous system relapse and leukoencephalopathy in patients receiving marrow transplants for acute leukemia. Blood 67 (1): 195-9, 1986.
- Oshima K, Kanda Y, Yamashita T, et al.: Central nervous system relapse of leukemia after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 14 (10): 1100-7, 2008.
- Ruutu T, Corradini P, Gratwohl A, et al.: Use of intrathecal prophylaxis in allogeneic haematopoietic stem cell transplantation for malignant blood diseases: a survey of the European Group for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant 35 (2): 121-4, 2005.
- Maude SL, Laetsch TW, Buechner J, et al.: Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med 378 (5): 439-448, 2018.
- Mehta J, Powles R, Treleaven J, et al.: Outcome of acute leukemia relapsing after bone marrow transplantation: utility of second transplants and adoptive immunotherapy. Bone Marrow Transplant 19 (7): 709-19, 1997.
- Kuhlen M, Willasch AM, Dalle JH, et al.: Outcome of relapse after allogeneic HSCT in children with ALL enrolled in the ALL-SCT 2003/2007 trial. Br J Haematol 180 (1): 82-89, 2018.
- Eapen M, Giralt SA, Horowitz MM, et al.: Second transplant for acute and chronic leukemia relapsing after first HLA-identical sibling transplant. Bone Marrow Transplant 34 (8): 721-7, 2004.
- Bosi A, Laszlo D, Labopin M, et al.: Second allogeneic bone marrow transplantation in acute leukemia: results of a survey by the European Cooperative Group for Blood and Marrow Transplantation. J Clin Oncol 19 (16): 3675-84, 2001.
- Willasch AM, Salzmann-Manrique E, Krenn T, et al.: Treatment of relapse after allogeneic stem cell transplantation in children and adolescents with ALL: the Frankfurt experience. Bone Marrow Transplant 52 (2): 201-208, 2017.
- Yaniv I, Krauss AC, Beohou E, et al.: Second Hematopoietic Stem Cell Transplantation for Post-Transplantation Relapsed Acute Leukemia in Children: A Retrospective EBMT-PDWP Study. Biol Blood Marrow Transplant 24 (8): 1629-1642, 2018.
- Nishikawa T, Inagaki J, Nagatoshi Y, et al.: The second therapeutic trial for children with hematological malignancies who relapsed after their first allogeneic SCT: long-term outcomes. Pediatr Transplant 16 (7): 722-8, 2012.
- Bajwa R, Schechter T, Soni S, et al.: Outcome of children who experience disease relapse following allogeneic hematopoietic SCT for hematologic malignancies. Bone Marrow Transplant 48 (5): 661-5, 2013.
- Schechter T, Avila L, Frangoul H, et al.: Effect of acute graft-versus-host disease on the outcome of second allogeneic hematopoietic stem cell transplant in children. Leuk Lymphoma 54 (1): 105-9, 2013.
- Pulsipher MA, Boucher KM, Wall D, et al.: Reduced-intensity allogeneic transplantation in pediatric patients ineligible for myeloablative therapy: results of the Pediatric Blood and Marrow Transplant Consortium Study ONC0313. Blood 114 (7): 1429-36, 2009.
- Collins RH, Goldstein S, Giralt S, et al.: Donor leukocyte infusions in acute lymphocytic leukemia. Bone Marrow Transplant 26 (5): 511-6, 2000.
- Levine JE, Barrett AJ, Zhang MJ, et al.: Donor leukocyte infusions to treat hematologic malignancy relapse following allo-SCT in a pediatric population. Bone Marrow Transplant 42 (3): 201-5, 2008.
- Bhadri VA, McGregor MR, Venn NC, et al.: Isolated testicular relapse after allo-SCT in boys with ALL: outcome without second transplant. Bone Marrow Transplant 45 (2): 397-9, 2010.
- von Stackelberg A, Locatelli F, Zugmaier G, et al.: Phase I/Phase II Study of Blinatumomab in Pediatric Patients With Relapsed/Refractory Acute Lymphoblastic Leukemia. J Clin Oncol 34 (36): 4381-4389, 2016.
- Queudeville M, Stein AS, Locatelli F, et al.: Low leukemia burden improves blinatumomab efficacy in patients with relapsed/refractory B-cell acute lymphoblastic leukemia. Cancer 129 (9): 1384-1393, 2023.
- Kantarjian H, Thomas D, Jorgensen J, et al.: Results of inotuzumab ozogamicin, a CD22 monoclonal antibody, in refractory and relapsed acute lymphocytic leukemia. Cancer 119 (15): 2728-36, 2013.
- Kantarjian HM, DeAngelo DJ, Stelljes M, et al.: Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia. N Engl J Med 375 (8): 740-53, 2016.
- Pennesi E, Michels N, Brivio E, et al.: Inotuzumab ozogamicin as single agent in pediatric patients with relapsed and refractory acute lymphoblastic leukemia: results from a phase II trial. Leukemia 36 (6): 1516-1524, 2022.
- Kebriaei P, Cutler C, de Lima M, et al.: Management of important adverse events associated with inotuzumab ozogamicin: expert panel review. Bone Marrow Transplant 53 (4): 449-456, 2018.
- Maury S, Chevret S, Thomas X, et al.: Rituximab in B-Lineage Adult Acute Lymphoblastic Leukemia. N Engl J Med 375 (11): 1044-53, 2016.
- Sasaki K, Kantarjian HM, Morita K, et al.: Hyper-CVAD plus ofatumumab versus hyper-CVAD plus rituximab as frontline therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: A propensity score analysis. Cancer 127 (18): 3381-3389, 2021.
- Grupp SA, Kalos M, Barrett D, et al.: Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 368 (16): 1509-18, 2013.
- Maude SL, Frey N, Shaw PA, et al.: Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371 (16): 1507-17, 2014.
- Fitzgerald JC, Weiss SL, Maude SL, et al.: Cytokine Release Syndrome After Chimeric Antigen Receptor T Cell Therapy for Acute Lymphoblastic Leukemia. Crit Care Med 45 (2): e124-e131, 2017.
- Kadauke S, Myers RM, Li Y, et al.: Risk-Adapted Preemptive Tocilizumab to Prevent Severe Cytokine Release Syndrome After CTL019 for Pediatric B-Cell Acute Lymphoblastic Leukemia: A Prospective Clinical Trial. J Clin Oncol 39 (8): 920-930, 2021.
- Shalabi H, Wolters PL, Martin S, et al.: Systematic Evaluation of Neurotoxicity in Children and Young Adults Undergoing CD22 Chimeric Antigen Receptor T-Cell Therapy. J Immunother 41 (7): 350-358, 2018.
- Gardner RA, Finney O, Annesley C, et al.: Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood 129 (25): 3322-3331, 2017.
- Levine JE, Grupp SA, Pulsipher MA, et al.: Pooled safety analysis of tisagenlecleucel in children and young adults with B cell acute lymphoblastic leukemia. J Immunother Cancer 9 (8): , 2021.
- McNerney KO, Si Lim SJ, Ishikawa K, et al.: HLH-like toxicities predict poor survival after the use of tisagenlecleucel in children and young adults with B-ALL. Blood Adv 7 (12): 2758-2771, 2023.
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al.: T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 385 (9967): 517-28, 2015.
- V Stackelberg A, Jäschke K, Jousseaume E, et al.: Tisagenlecleucel vs. historical standard of care in children and young adult patients with relapsed/refractory B-cell precursor acute lymphoblastic leukemia. Leukemia 37 (12): 2346-2355, 2023.
- Pasquini MC, Hu ZH, Curran K, et al.: Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-Hodgkin lymphoma. Blood Adv 4 (21): 5414-5424, 2020.
- Ghorashian S, Jacoby E, De Moerloose B, et al.: Tisagenlecleucel therapy for relapsed or refractory B-cell acute lymphoblastic leukaemia in infants and children younger than 3 years of age at screening: an international, multicentre, retrospective cohort study. Lancet Haematol 9 (10): e766-e775, 2022.
- Laetsch TW, Maude SL, Rives S, et al.: Three-Year Update of Tisagenlecleucel in Pediatric and Young Adult Patients With Relapsed/Refractory Acute Lymphoblastic Leukemia in the ELIANA Trial. J Clin Oncol 41 (9): 1664-1669, 2023.
- Shah NN, Lee DW, Yates B, et al.: Long-Term Follow-Up of CD19-CAR T-Cell Therapy in Children and Young Adults With B-ALL. J Clin Oncol 39 (15): 1650-1659, 2021.
- Curran KJ, Margossian SP, Kernan NA, et al.: Toxicity and response after CD19-specific CAR T-cell therapy in pediatric/young adult relapsed/refractory B-ALL. Blood 134 (26): 2361-2368, 2019.
- Myers RM, Li Y, Barz Leahy A, et al.: Humanized CD19-Targeted Chimeric Antigen Receptor (CAR) T Cells in CAR-Naive and CAR-Exposed Children and Young Adults With Relapsed or Refractory Acute Lymphoblastic Leukemia. J Clin Oncol 39 (27): 3044-3055, 2021.
- Leahy AB, Newman H, Li Y, et al.: CD19-targeted chimeric antigen receptor T-cell therapy for CNS relapsed or refractory acute lymphocytic leukaemia: a post-hoc analysis of pooled data from five clinical trials. Lancet Haematol 8 (10): e711-e722, 2021.
- Fabrizio VA, Phillips CL, Lane A, et al.: Tisagenlecleucel outcomes in relapsed/refractory extramedullary ALL: a Pediatric Real World CAR Consortium Report. Blood Adv 6 (2): 600-610, 2022.
- Jacoby E, Ghorashian S, Vormoor B, et al.: CD19 CAR T-cells for pediatric relapsed acute lymphoblastic leukemia with active CNS involvement: a retrospective international study. Leukemia 36 (6): 1525-1532, 2022.
- Myers RM, Taraseviciute A, Steinberg SM, et al.: Blinatumomab Nonresponse and High-Disease Burden Are Associated With Inferior Outcomes After CD19-CAR for B-ALL. J Clin Oncol 40 (9): 932-944, 2022.
- Holland EM, Yates B, Ling A, et al.: Characterization of extramedullary disease in B-ALL and response to CAR T-cell therapy. Blood Adv 6 (7): 2167-2182, 2022.
- Leahy AB, Devine KJ, Li Y, et al.: Impact of high-risk cytogenetics on outcomes for children and young adults receiving CD19-directed CAR T-cell therapy. Blood 139 (14): 2173-2185, 2022.
- Schultz LM, Baggott C, Prabhu S, et al.: Disease Burden Affects Outcomes in Pediatric and Young Adult B-Cell Lymphoblastic Leukemia After Commercial Tisagenlecleucel: A Pediatric Real-World Chimeric Antigen Receptor Consortium Report. J Clin Oncol 40 (9): 945-955, 2022.
- Pulsipher MA, Han X, Maude SL, et al.: Next-Generation Sequencing of Minimal Residual Disease for Predicting Relapse after Tisagenlecleucel in Children and Young Adults with Acute Lymphoblastic Leukemia. Blood Cancer Discov 3 (1): 66-81, 2022.
- Stefanski HE, Eaton A, Baggott C, et al.: Higher doses of tisagenlecleucel are associated with improved outcomes: a report from the pediatric real-world CAR consortium. Blood Adv 7 (4): 541-548, 2023.
- Bader P, Rossig C, Hutter M, et al.: CD19 CAR T cells are an effective therapy for posttransplant relapse in patients with B-lineage ALL: real-world data from Germany. Blood Adv 7 (11): 2436-2448, 2023.
- Lamble AJ, Myers RM, Taraseviciute A, et al.: Preinfusion factors impacting relapse immunophenotype following CD19 CAR T cells. Blood Adv 7 (4): 575-585, 2023.
- Schultz LM, Eaton A, Baggott C, et al.: Outcomes After Nonresponse and Relapse Post-Tisagenlecleucel in Children, Adolescents, and Young Adults With B-Cell Acute Lymphoblastic Leukemia. J Clin Oncol 41 (2): 354-363, 2023.
- Del Bufalo F, Becilli M, Rosignoli C, et al.: Allogeneic, donor-derived, second-generation, CD19-directed CAR-T cells for the treatment of pediatric relapsed/refractory BCP-ALL. Blood 142 (2): 146-157, 2023.
- Summers C, Wu QV, Annesley C, et al.: Hematopoietic Cell Transplantation after CD19 Chimeric Antigen Receptor T Cell-Induced Acute Lymphoblastic Lymphoma Remission Confers a Leukemia-Free Survival Advantage. Transplant Cell Ther 28 (1): 21-29, 2022.
- Zhang Y, Chen H, Song Y, et al.: Chimeric antigens receptor T cell therapy as a bridge to haematopoietic stem cell transplantation for refractory/ relapsed B-cell acute lymphomalastic leukemia. Br J Haematol 189 (1): 146-152, 2020.
- Sotillo E, Barrett DM, Black KL, et al.: Convergence of Acquired Mutations and Alternative Splicing of CD19 Enables Resistance to CART-19 Immunotherapy. Cancer Discov 5 (12): 1282-95, 2015.
- Fry TJ, Shah NN, Orentas RJ, et al.: CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med 24 (1): 20-28, 2018.
- Pan J, Niu Q, Deng B, et al.: CD22 CAR T-cell therapy in refractory or relapsed B acute lymphoblastic leukemia. Leukemia 33 (12): 2854-2866, 2019.
- Hu GH, Zhao XY, Zuo YX, et al.: Unmanipulated haploidentical hematopoietic stem cell transplantation is an excellent option for children and young adult relapsed/refractory Philadelphia chromosome-negative B-cell acute lymphoblastic leukemia after CAR-T-cell therapy. Leukemia 35 (11): 3092-3100, 2021.
- Shah NN, Highfill SL, Shalabi H, et al.: CD4/CD8 T-Cell Selection Affects Chimeric Antigen Receptor (CAR) T-Cell Potency and Toxicity: Updated Results From a Phase I Anti-CD22 CAR T-Cell Trial. J Clin Oncol 38 (17): 1938-1950, 2020.
- Wang T, Tang Y, Cai J, et al.: Coadministration of CD19- and CD22-Directed Chimeric Antigen Receptor T-Cell Therapy in Childhood B-Cell Acute Lymphoblastic Leukemia: A Single-Arm, Multicenter, Phase II Trial. J Clin Oncol 41 (9): 1670-1683, 2023.
- Pan J, Tang K, Luo Y, et al.: Sequential CD19 and CD22 chimeric antigen receptor T-cell therapy for childhood refractory or relapsed B-cell acute lymphocytic leukaemia: a single-arm, phase 2 study. Lancet Oncol 24 (11): 1229-1241, 2023.
- Spiegel JY, Patel S, Muffly L, et al.: CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nat Med 27 (8): 1419-1431, 2021.
- Cordoba S, Onuoha S, Thomas S, et al.: CAR T cells with dual targeting of CD19 and CD22 in pediatric and young adult patients with relapsed or refractory B cell acute lymphoblastic leukemia: a phase 1 trial. Nat Med 27 (10): 1797-1805, 2021.
- Shalabi H, Qin H, Su A, et al.: CD19/22 CAR T cells in children and young adults with B-ALL: phase 1 results and development of a novel bicistronic CAR. Blood 140 (5): 451-463, 2022.
- Möricke A, Zimmermann M, Reiter A, et al.: Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia 24 (2): 265-84, 2010.
- Silverman LB, Stevenson KE, O'Brien JE, et al.: Long-term results of Dana-Farber Cancer Institute ALL Consortium protocols for children with newly diagnosed acute lymphoblastic leukemia (1985-2000). Leukemia 24 (2): 320-34, 2010.
- Pui CH, Pei D, Sandlund JT, et al.: Long-term results of St Jude Total Therapy Studies 11, 12, 13A, 13B, and 14 for childhood acute lymphoblastic leukemia. Leukemia 24 (2): 371-82, 2010.
- Ritchey AK, Pollock BH, Lauer SJ, et al.: Improved survival of children with isolated CNS relapse of acute lymphoblastic leukemia: a pediatric oncology group study . J Clin Oncol 17 (12): 3745-52, 1999.
- Domenech C, Mercier M, Plouvier E, et al.: First isolated extramedullary relapse in children with B-cell precursor acute lymphoblastic leukaemia: results of the Cooprall-97 study. Eur J Cancer 44 (16): 2461-9, 2008.
- Hagedorn N, Acquaviva C, Fronkova E, et al.: Submicroscopic bone marrow involvement in isolated extramedullary relapses in childhood acute lymphoblastic leukemia: a more precise definition of "isolated" and its possible clinical implications, a collaborative study of the Resistant Disease Committee of the International BFM study group. Blood 110 (12): 4022-9, 2007.
- Ribeiro RC, Rivera GK, Hudson M, et al.: An intensive re-treatment protocol for children with an isolated CNS relapse of acute lymphoblastic leukemia. J Clin Oncol 13 (2): 333-8, 1995.
- Kumar P, Kun LE, Hustu HO, et al.: Survival outcome following isolated central nervous system relapse treated with additional chemotherapy and craniospinal irradiation in childhood acute lymphoblastic leukemia. Int J Radiat Oncol Biol Phys 31 (3): 477-83, 1995.
- Hastings C, Chen Y, Devidas M, et al.: Late isolated central nervous system relapse in childhood B-cell acute lymphoblastic leukemia treated with intensified systemic therapy and delayed reduced dose cranial radiation: A report from the Children's Oncology Group study AALL02P2. Pediatr Blood Cancer 68 (12): e29256, 2021.
- Yoshihara T, Morimoto A, Kuroda H, et al.: Allogeneic stem cell transplantation in children with acute lymphoblastic leukemia after isolated central nervous system relapse: our experiences and review of the literature. Bone Marrow Transplant 37 (1): 25-31, 2006.
- Harker-Murray PD, Thomas AJ, Wagner JE, et al.: Allogeneic hematopoietic cell transplantation in children with relapsed acute lymphoblastic leukemia isolated to the central nervous system. Biol Blood Marrow Transplant 14 (6): 685-92, 2008.
- Eapen M, Zhang MJ, Devidas M, et al.: Outcomes after HLA-matched sibling transplantation or chemotherapy in children with acute lymphoblastic leukemia in a second remission after an isolated central nervous system relapse: a collaborative study of the Children's Oncology Group and the Center for International Blood and Marrow Transplant Research. Leukemia 22 (2): 281-6, 2008.
- Wofford MM, Smith SD, Shuster JJ, et al.: Treatment of occult or late overt testicular relapse in children with acute lymphoblastic leukemia: a Pediatric Oncology Group study. J Clin Oncol 10 (4): 624-30, 1992.
- Trigg ME, Steinherz PG, Chappell R, et al.: Early testicular biopsy in males with acute lymphoblastic leukemia: lack of impact on subsequent event-free survival. J Pediatr Hematol Oncol 22 (1): 27-33, 2000 Jan-Feb.
- van den Berg H, Langeveld NE, Veenhof CH, et al.: Treatment of isolated testicular recurrence of acute lymphoblastic leukemia without radiotherapy. Report from the Dutch Late Effects Study Group. Cancer 79 (11): 2257-62, 1997.
- Barredo JC, Hastings C, Lu X, et al.: Isolated late testicular relapse of B-cell acute lymphoblastic leukemia treated with intensive systemic chemotherapy and response-based testicular radiation: A Children's Oncology Group study. Pediatr Blood Cancer 65 (5): e26928, 2018.
Latest Updates to This Summary (08 / 28 / 2024)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Central Nervous System (CNS)-Directed Therapy for Childhood ALL
Added text to state that in a Pediatric Normal Tissue Effects in the Clinic report on subsequent malignancies, patients who received radiation therapy to the brain had a pooled excess relative ratio per Gy of 0.44 for subsequent meningiomas. Patients treated with 12 Gy of radiation therapy have a substantially lower potential for developing meningiomas than those treated with 24 Gy (cited Casey et al. as reference 33).
Postinduction Treatment for Specific ALL Subgroups
Added text to state that among all patients in the analysis cohort of the AALL0232 trial, patients with osteonecrosis had superior 5-year event-free survival and overall survival rates than patients without osteonecrosis. Similar differences were seen in patients older than 10 years. Improved survival was directly attributed to reduced relapse rates (cited Mattano et al. as reference 67).
Treatment of Relapsed Childhood ALL
Added text about the results of a substudy of the FORUM trial that compared two chemotherapy preparative regimens in 191 children who were nonrandomly assigned to non–total-body irradiation (TBI) regimens. The authors concluded that there is strong evidence that TBI-based regimens are superior in children older than 2 years, and this result should be considered as parents and clinicians evaluate the risks and benefits of TBI in children aged 2 to 4 years (cited Bader et al. as reference 93).
This summary is written and maintained by the
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of childhood acute lymphoblastic leukemia. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Childhood Acute Lymphoblastic Leukemia Treatment are:
- William L. Carroll, MD (Laura and Isaac Perlmutter Cancer Center at NYU Langone)
- Alan Scott Gamis, MD, MPH (Children's Mercy Hospital)
- Michelle Hermiston, MD, PhD (University of California, San Francisco)
- Karen J. Marcus, MD, FACR (Dana-Farber Cancer Institute/Boston Children's Hospital)
- Jessica Pollard, MD (Dana-Farber/Boston Children's Cancer and Blood Disorders Center)
- Michael A. Pulsipher, MD (Huntsman Cancer Institute at University of Utah)
- Rachel E. Rau, MD (University of Washington School of Medicine, Seatle Children's)
- Arthur Kim Ritchey, MD (Children's Hospital of Pittsburgh of UPMC)
- Lewis B. Silverman, MD (Dana-Farber Cancer Institute/Boston Children's Hospital)
- Malcolm A. Smith, MD, PhD (National Cancer Institute)
- Sarah K. Tasian, MD (Children's Hospital of Philadelphia)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Pediatric Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as "NCI's PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary]."
The preferred citation for this PDQ summary is:
PDQ® Pediatric Treatment Editorial Board. PDQ Childhood Acute Lymphoblastic Leukemia Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at:
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either "standard" or "under clinical evaluation." These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our
Last Revised: 2024-08-28
This information does not replace the advice of a doctor. Ignite Healthwise, LLC, disclaims any warranty or liability for your use of this information. Your use of this information means that you agree to the
Healthwise, Healthwise for every health decision, and the Healthwise logo are trademarks of Ignite Healthwise, LLC.
Page Footer
I want to...
Audiences
Secure Member Sites
The Cigna Group Information
Disclaimer
Individual and family medical and dental insurance plans are insured by Cigna Health and Life Insurance Company (CHLIC), Cigna HealthCare of Arizona, Inc., Cigna HealthCare of Illinois, Inc., Cigna HealthCare of Georgia, Inc., Cigna HealthCare of North Carolina, Inc., Cigna HealthCare of South Carolina, Inc., and Cigna HealthCare of Texas, Inc. Group health insurance and health benefit plans are insured or administered by CHLIC, Connecticut General Life Insurance Company (CGLIC), or their affiliates (see
All insurance policies and group benefit plans contain exclusions and limitations. For availability, costs and complete details of coverage, contact a licensed agent or Cigna sales representative. This website is not intended for residents of New Mexico.