Treatment Option Overview
There are different types of treatment for children with colorectal cancer.
Some treatments are standard (the currently used treatment), and some are being tested in clinical trials. A treatment clinical trial is a research study meant to help improve current treatments or obtain information on new treatments for patients with cancer. When clinical trials show that a new treatment is better than the standard treatment, the new treatment may become the standard treatment.
Because cancer in children is rare, taking part in a clinical trial should be considered. Some clinical trials are open only to patients who have not started treatment.
Children with colorectal cancer should have their treatment planned by a team of doctors who are experts in treating childhood cancer.
Treatment will be overseen by a pediatric oncologist, a doctor who specializes in treating children with cancer. The pediatric oncologist works with other pediatric health professionals who are experts in treating children with cancer and who specialize in certain areas of medicine. This may include the following specialists and others:
- Pediatrician.
- Pediatric gastroenterologist.
- Pediatric surgeon.
- Radiation oncologist.
- Pathologist.
- Pediatric nurse specialist.
- Social worker.
- Rehabilitation specialist.
- Psychologist.
- Child-life specialist.
Four types of standard treatment are used:
Surgery
Surgery to remove the cancer is done if the cancer has not spread to other parts of the body at diagnosis.
Radiation therapy
Radiation therapy is a cancer treatment that uses high-energy x-rays or other types of radiation to kill cancer cells or keep them from growing. External radiation therapy uses a machine outside the body to send radiation toward the area of the body with cancer.
Chemotherapy
Chemotherapy is a cancer treatment that uses drugs to stop the growth of cancer cells, either by killing the cells or by stopping them from dividing. When chemotherapy is taken by mouth or injected into a vein or muscle, the drugs enter the bloodstream and can reach cancer cells throughout the body (systemic chemotherapy). Chemotherapy using more than one drug is called combination chemotherapy.
Immunotherapy
Immunotherapy is a treatment that uses the patient's immune system to fight cancer. Substances made by the body or made in a laboratory are used to boost, direct, or restore the body's natural defenses against cancer. This cancer treatment is a type of biologic therapy.
Immune checkpoint inhibitor therapy is a type of immunotherapy. Some types of immune cells, such as T cells, and some cancer cells have certain proteins, called checkpoint proteins, on their surface that keep immune responses in check. When cancer cells have large amounts of these proteins, they will not be attacked and killed by T cells. Immune checkpoint inhibitors block these proteins and the ability of T cells to kill cancer cells is increased.
There are two types of immune checkpoint inhibitor therapy:
- PD-1 and PD-L1 inhibitor therapy: PD-1 is a protein on the surface of T cells that helps keep the body's immune responses in check. PD-L1 is a protein found on some types of cancer cells. When PD-1 attaches to PD-L1, it stops the T cell from killing the cancer cell. PD-1 and PD-L1 inhibitors keep PD-1 and PD-L1 proteins from attaching to each other. This allows the T cells to kill cancer cells. Nivolumab is a type of PD-1 inhibitor that has been used to treat children 12 years and older with progressive colorectal cancer.
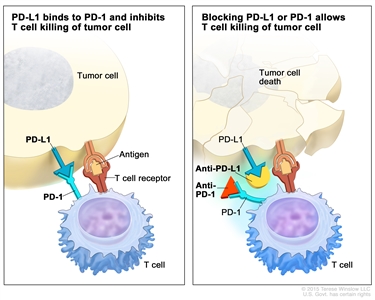
Immune checkpoint inhibitor. Checkpoint proteins, such as PD-L1 on tumor cells and PD-1 on T cells, help keep immune responses in check. The binding of PD-L1 to PD-1 keeps T cells from killing tumor cells in the body (left panel). Blocking the binding of PD-L1 to PD-1 with an immune checkpoint inhibitor (anti-PD-L1 or anti-PD-1) allows the T cells to kill tumor cells (right panel).
- CTLA-4 inhibitor. CTLA-4 is a protein on the surface of T cells that helps keep the body's immune responses in check. When CTLA-4 attaches to another protein called B7 on a cancer cell, it stops the T cell from killing the cancer cell. CTLA-4 inhibitors attach to CTLA-4 and allow the T cells to kill cancer cells. Ipilimumab is used to treat children 12 years and older with progressive colorectal cancer.
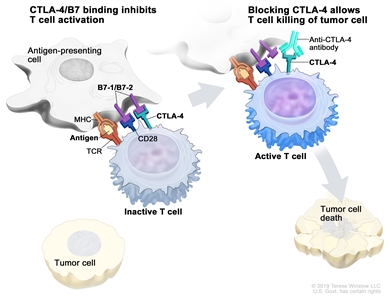
Immune checkpoint inhibitor. Checkpoint proteins, such as B7-1/B7-2 on antigen-presenting cells (APC) and CTLA-4 on T cells, help keep the body's immune responses in check. When the T-cell receptor (TCR) binds to antigen and major histocompatibility complex (MHC) proteins on the APC and CD28 binds to B7-1/B7-2 on the APC, the T cell can be activated. However, the binding of B7-1/B7-2 to CTLA-4 keeps the T cells in the inactive state so they are not able to kill tumor cells in the body (left panel). Blocking the binding of B7-1/B7-2 to CTLA-4 with an immune checkpoint inhibitor (anti-CTLA-4 antibody) allows the T cells to be active and to kill tumor cells (right panel).
New types of treatment are being tested in clinical trials.
Information about clinical trials is available from the NCI website.
Treatment for childhood colorectal cancer may cause side effects.
To learn more about side effects that begin during treatment for cancer, visit Side Effects.
Side effects from cancer treatment that begin after treatment and continue for months or years are called late effects. Late effects of cancer treatment may include the following:
- Physical problems.
- Changes in mood, feelings, thinking, learning, or memory.
- Second cancers (new types of cancer) or other conditions.
Some late effects may be treated or controlled. It is important to talk with your child's doctors about the possible late effects caused by some treatments. See the PDQ summary on Late Effects of Treatment for Childhood Cancer for more information.
Patients may want to think about taking part in a clinical trial.
For some patients, taking part in a clinical trial may be the best treatment choice. Clinical trials are part of the cancer research process. Clinical trials are done to find out if new cancer treatments are safe and effective or better than the standard treatment.
Many of today's standard treatments for cancer are based on earlier clinical trials. Patients who take part in a clinical trial may receive the standard treatment or be among the first to receive a new treatment.
Patients who take part in clinical trials also help improve the way cancer will be treated in the future. Even when clinical trials do not lead to effective new treatments, they often answer important questions and help move research forward.
Patients can enter clinical trials before, during, or after starting their cancer treatment.
Some clinical trials only include patients who have not yet received treatment. Other trials test treatments for patients whose cancer has not gotten better. There are also clinical trials that test new ways to stop cancer from recurring (coming back) or reduce the side effects of cancer treatment.
Clinical trials are taking place in many parts of the country. Information about clinical trials supported by NCI can be found on NCI's clinical trials search webpage. Clinical trials supported by other organizations can be found on the ClinicalTrials.gov website.
Follow-up tests may be needed.
As your child goes through treatment, they will have follow-up tests or check-ups. Some tests that were done to diagnose or stage the cancer may be repeated to see how well the treatment is working. Decisions about whether to continue, change, or stop treatment may be based on the results of these tests.
Some of the tests will continue to be done from time to time after treatment has ended. The results of these tests can show if your child's condition has changed or if the cancer has recurred (come back).