Ovarian Borderline Tumors Treatment (PDQ®): Treatment - Health Professional Information [NCI]
General Information About Ovarian Borderline Tumors
Incidence and Mortality
Ovarian borderline tumors (i.e., tumors of low malignant potential) account for 15% of all epithelial ovarian cancers. Nearly 75% of borderline tumors are stage I at the time of diagnosis.[
A review of 22 series (which included 953 patients), with a mean follow-up of 7 years, revealed a survival rate of 92% for patients with advanced-stage ovarian borderline tumors, if patients with so-called invasive implants were excluded. The causes of death in these patients were determined to be benign complications of disease (e.g., small bowel obstruction), complications of therapy, and only rarely (0.7% of patients), malignant transformation.[
Another large retrospective study showed that early stage, serous histology, and younger age are associated with a more favorable prognosis in patients with ovarian borderline tumors.[
Endometrioid Tumors
Endometrioid borderline tumors are less common and should not be regarded as malignant because they seldom, if ever, metastasize. However, malignant transformation can occur and may be associated with a similar tumor outside of the ovary. Such tumors are the result of either a second primary or rupture of the primary endometrial tumor.[
References:
- Berek JS, Renz M, Kehoe S, et al.: Cancer of the ovary, fallopian tube, and peritoneum: 2021 update. Int J Gynaecol Obstet 155 (Suppl 1): 61-85, 2021.
- Kurman RJ, Trimble CL: The behavior of serous tumors of low malignant potential: are they ever malignant? Int J Gynecol Pathol 12 (2): 120-7, 1993.
- Leake JF, Currie JL, Rosenshein NB, et al.: Long-term follow-up of serous ovarian tumors of low malignant potential. Gynecol Oncol 47 (2): 150-8, 1992.
- Zanetta G, Rota S, Chiari S, et al.: Behavior of borderline tumors with particular interest to persistence, recurrence, and progression to invasive carcinoma: a prospective study. J Clin Oncol 19 (10): 2658-64, 2001.
- Kaern J, Tropé CG, Abeler VM: A retrospective study of 370 borderline tumors of the ovary treated at the Norwegian Radium Hospital from 1970 to 1982. A review of clinicopathologic features and treatment modalities. Cancer 71 (5): 1810-20, 1993.
- Norris HJ: Proliferative endometrioid tumors and endometrioid tumors of low malignant potential of the ovary. Int J Gynecol Pathol 12 (2): 134-40, 1993.
Stage Information for Ovarian Borderline Tumors
Fédération Internationale de Gynécologie et d'Obstétrique (FIGO) Staging
FIGO and the American Joint Committee on Cancer have designated staging to define ovarian borderline tumors; the FIGO system is most commonly used.[
| Stage | Definition | Illustration |
|---|---|---|
| FIGO = Fédération Internationale de Gynécologie et d'Obstétrique. | ||
| a Adapted from FIGO Committee for Gynecologic Oncology.[ |
||
| I | Tumor confined to ovaries or fallopian tube(s). | 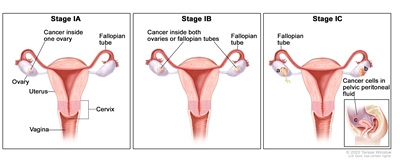 |
| IA | Tumor limited to one ovary (capsule intact) or fallopian tube; no tumor on ovarian or fallopian tube surface; no malignant cells in the ascites or peritoneal washings. | |
| IB | Tumor limited to both ovaries (capsules intact) or fallopian tubes; no tumor on ovarian or fallopian tube surface; no malignant cells in the ascites or peritoneal washings. | |
| IC | Tumor limited to one or both ovaries or fallopian tubes, with any of the following: | |
| IC1: Surgical spill. | ||
| IC2: Capsule ruptured before surgery or tumor on ovarian or fallopian tube surface. | ||
| IC3: Malignant cells in the ascites or peritoneal washings. | ||
| Stage | Definition | Illustration |
|---|---|---|
| FIGO = Fédération Internationale de Gynécologie et d'Obstétrique. | ||
| a Adapted from FIGO Committee for Gynecologic Oncology.[ |
||
| II | Tumor involves one or both ovaries or fallopian tubes with pelvic extension (below the pelvic brim) or primary peritoneal cancer. | 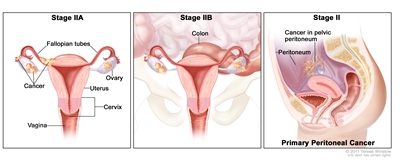 |
| IIA | Extension and/or implants on uterus and/or fallopian tubes and/or ovaries. | |
| IIB | Extension to other pelvic intraperitoneal tissues. | |
| Stage | Definition | Illustration |
|---|---|---|
| FIGO = Fédération Internationale de Gynécologie et d'Obstétrique. | ||
| a Adapted from FIGO Committee for Gynecologic Oncology.[ |
||
| III | Tumor involves one or both ovaries or fallopian tubes, or primary peritoneal cancer, with cytologically or histologically confirmed spread to the peritoneum outside the pelvis and/or metastasis to the retroperitoneal lymph nodes. | |
| IIIA1 | Positive retroperitoneal lymph nodes only (cytologically or histologically proven): | 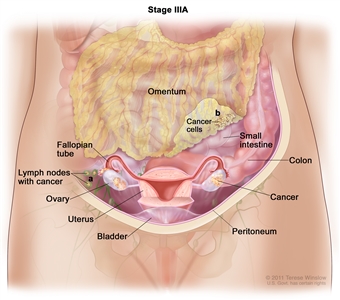 |
| IIIA1(I): Metastasis ≤10 mm in greatest dimension. | ||
| IIIA1(ii): Metastasis >10 mm in greatest dimension. | ||
| IIIA2 | Microscopic extrapelvic (above the pelvic brim) peritoneal involvement with or without positive retroperitoneal lymph nodes. | |
| IIIB | Macroscopic peritoneal metastasis beyond the pelvis ≤2 cm in greatest dimension, with or without metastasis to the retroperitoneal lymph nodes. | 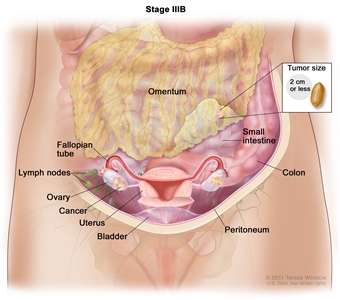 |
| IIIC | Macroscopic peritoneal metastasis beyond the pelvis >2 cm in greatest dimension, with or without metastasis to the retroperitoneal lymph nodes (includes extension of tumor to capsule of liver and spleen without parenchymal involvement of either organ). | 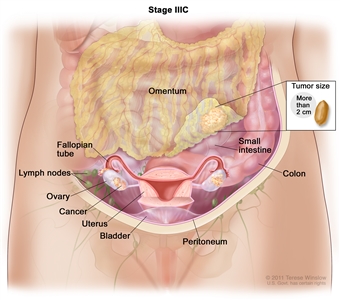 |
| Stage | Definition | Illustration |
|---|---|---|
| FIGO = Fédération Internationale de Gynécologie et d'Obstétrique. | ||
| a Adapted from FIGO Committee for Gynecologic Oncology.[ |
||
| IV | Distant metastasis excluding peritoneal metastases. | 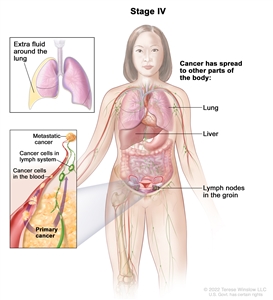 |
| IVA | Pleural effusion with positive cytology. | |
| IVB | Parenchymal metastases and metastases to extra-abdominal organs (including inguinal lymph nodes and lymph nodes outside of the abdominal cavity). | |
References:
- Berek JS, Renz M, Kehoe S, et al.: Cancer of the ovary, fallopian tube, and peritoneum: 2021 update. Int J Gynaecol Obstet 155 (Suppl 1): 61-85, 2021.
- Ovary, fallopian tube, and primary peritoneal carcinoma. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp 681-90.
Treatment of Early-Stage Ovarian Borderline Tumors
Treatment Options for Early-Stage Ovarian Borderline Tumors
Treatment options for early-stage ovarian borderline tumors include the following:
-
SurgerySurgery .
Surgery
In early-stage disease (stage I or II), no additional treatment is indicated for patients with a completely resected borderline tumor.[
Importance of Staging for Treatment
The value of complete staging has not been demonstrated for patients with early-stage cases, but the opposite ovary should be carefully evaluated for evidence of bilateral disease. Although the impact of surgical staging on therapeutic management is not defined, in a study of 29 patients with presumed localized disease, 7 patients were upstaged following complete surgical staging.[
In two other studies, 16% and 18% of patients with presumed localized borderline tumors were upstaged as a result of a staging laparotomy.[
In another study, patients with localized intraperitoneal disease and negative lymph nodes had a low incidence of recurrence (5%), whereas patients with localized intraperitoneal disease and positive lymph nodes had a statistically significant higher incidence of recurrence (50%).[
Fertility Preservation
When a patient wishes to retain childbearing potential, a unilateral salpingo-oophorectomy is adequate therapy.[
In a large series, the relapse rate was higher for patients who underwent more conservative surgery (cystectomy > unilateral oophorectomy > total abdominal hysterectomy and bilateral salpingo-oophorectomy [TAHBSO]). However, differences were not statistically significant, and survival was nearly 100% for all groups.[
Current Clinical Trials
Use our
References:
- Tropé C, Kaern J, Vergote IB, et al.: Are borderline tumors of the ovary overtreated both surgically and systemically? A review of four prospective randomized trials including 253 patients with borderline tumors. Gynecol Oncol 51 (2): 236-43, 1993.
- Yazigi R, Sandstad J, Munoz AK: Primary staging in ovarian tumors of low malignant potential. Gynecol Oncol 31 (3): 402-8, 1988.
- Snider DD, Stuart GC, Nation JG, et al.: Evaluation of surgical staging in stage I low malignant potential ovarian tumors. Gynecol Oncol 40 (2): 129-32, 1991.
- Leake JF, Rader JS, Woodruff JD, et al.: Retroperitoneal lymphatic involvement with epithelial ovarian tumors of low malignant potential. Gynecol Oncol 42 (2): 124-30, 1991.
- Leake JF, Currie JL, Rosenshein NB, et al.: Long-term follow-up of serous ovarian tumors of low malignant potential. Gynecol Oncol 47 (2): 150-8, 1992.
- Kaern J, Tropé CG, Abeler VM: A retrospective study of 370 borderline tumors of the ovary treated at the Norwegian Radium Hospital from 1970 to 1982. A review of clinicopathologic features and treatment modalities. Cancer 71 (5): 1810-20, 1993.
- Lim-Tan SK, Cajigas HE, Scully RE: Ovarian cystectomy for serous borderline tumors: a follow-up study of 35 cases. Obstet Gynecol 72 (5): 775-81, 1988.
- Rice LW, Berkowitz RS, Mark SD, et al.: Epithelial ovarian tumors of borderline malignancy. Gynecol Oncol 39 (2): 195-8, 1990.
- Piura B, Dgani R, Blickstein I, et al.: Epithelial ovarian tumors of borderline malignancy: a study of 50 cases. Int J Gynecol Cancer 2 (4): 189-197, 1992.
- Casey AC, Bell DA, Lage JM, et al.: Epithelial ovarian tumors of borderline malignancy: long-term follow-up. Gynecol Oncol 50 (3): 316-22, 1993.
Treatment of Advanced-Stage Ovarian Borderline Tumors
Treatment Options for Advanced-Stage Ovarian Borderline Tumors
Treatment options for advanced-stage ovarian borderline tumors include the following:
-
SurgerySurgery .
Surgery
Patients with advanced disease should undergo a total hysterectomy, bilateral salpingo-oophorectomy, omentectomy, node sampling, and aggressive cytoreductive surgery. Patients with stage III or IV disease with no gross residual tumor had a 100% survival rate in some series regardless of the follow-up duration.[
Chemotherapy and/or radiation therapy are not indicated for patients with more advanced-stage disease and microscopic or gross residual disease. Scant evidence exists that postoperative chemotherapy or radiation therapy alters the course of this disease in any beneficial way.[
In a review of 150 patients with borderline ovarian tumors, the survival of patients with a residual tumor of less than 2 cm was significantly better than survival for those with a residual tumor from 2 cm to 5 cm or more than 5 cm (P < .05).[
Current Clinical Trials
Use our
References:
- Barnhill D, Heller P, Brzozowski P, et al.: Epithelial ovarian carcinoma of low malignant potential. Obstet Gynecol 65 (1): 53-9, 1985.
- Bostwick DG, Tazelaar HD, Ballon SC, et al.: Ovarian epithelial tumors of borderline malignancy. A clinical and pathologic study of 109 cases. Cancer 58 (9): 2052-65, 1986.
- Leake JF, Currie JL, Rosenshein NB, et al.: Long-term follow-up of serous ovarian tumors of low malignant potential. Gynecol Oncol 47 (2): 150-8, 1992.
- Casey AC, Bell DA, Lage JM, et al.: Epithelial ovarian tumors of borderline malignancy: long-term follow-up. Gynecol Oncol 50 (3): 316-22, 1993.
- Tumors of the ovary: neoplasms derived from coelomic epithelium. In: Morrow CP, Curtin JP: Synopsis of Gynecologic Oncology. 5th ed. Churchill Livingstone, 1998, pp 233-281.
- Sutton GP, Bundy BN, Omura GA, et al.: Stage III ovarian tumors of low malignant potential treated with cisplatin combination therapy (a Gynecologic Oncology Group study). Gynecol Oncol 41 (3): 230-3, 1991.
- Kaern J, Tropé CG, Abeler VM: A retrospective study of 370 borderline tumors of the ovary treated at the Norwegian Radium Hospital from 1970 to 1982. A review of clinicopathologic features and treatment modalities. Cancer 71 (5): 1810-20, 1993.
- Tamakoshi K, Kikkawa F, Nakashima N, et al.: Clinical behavior of borderline ovarian tumors: a study of 150 cases. J Surg Oncol 64 (2): 147-52, 1997.
- Bell DA, Scully RE: Serous borderline tumors of the peritoneum. Am J Surg Pathol 14 (3): 230-9, 1990.
- Michael H, Roth LM: Invasive and noninvasive implants in ovarian serous tumors of low malignant potential. Cancer 57 (6): 1240-7, 1986.
- Friedlander ML, Hedley DW, Swanson C, et al.: Prediction of long-term survival by flow cytometric analysis of cellular DNA content in patients with advanced ovarian cancer. J Clin Oncol 6 (2): 282-90, 1988.
- Kaern J, Trope C, Kjorstad KE, et al.: Cellular DNA content as a new prognostic tool in patients with borderline tumors of the ovary. Gynecol Oncol 38 (3): 452-7, 1990.
- de Nictolis M, Montironi R, Tommasoni S, et al.: Serous borderline tumors of the ovary. A clinicopathologic, immunohistochemical, and quantitative study of 44 cases. Cancer 70 (1): 152-60, 1992.
- Eltabbakh GH, Belinson JL, Kennedy AW, et al.: p53 and HER-2/neu overexpression in ovarian borderline tumors. Gynecol Oncol 65 (2): 218-24, 1997.
Treatment of Low-Grade Serous Carcinoma
Treatment Options for Low-Grade Serous Carcinoma
Low-grade serous carcinoma (LGSC), also known as invasive micropapillary serous carcinoma, arises either from serous borderline tumors or de novo. These tumors are uncommon, making up 5% of ovarian carcinomas, and occur in younger women. They have a better clinical prognosis than high-grade serous carcinomas. Molecular characterization of LGSC shows lower frequency of TP53 mutations, greater expression of estrogen and progesterone receptors, and a high prevalence of BRAF and NRAS mutations.[
Treatment approaches for patients with recurrent disease can include cytoreductive surgery, cytotoxic chemotherapy, hormonal therapy, or targeted agents.
Treatment options for LGSC include the following:
-
Surgery with or without chemotherapySurgery with or without chemotherapy . -
Secondary cytoreductive surgerySecondary cytoreductive surgery . -
Targeted therapiesTargeted therapies . - Hormone therapy alone or combined with chemotherapy (under clinical evaluation [NCT04095364]).
Surgery with or without chemotherapy
While complete cytoreductive surgery is a major component of the treatment approach to LGSC, these tumors tend to be chemoresistant.[
Secondary cytoreductive surgery
Evidence (secondary cytoreductive surgery):
- A single institution retrospective review evaluated secondary cytoreductive surgery performed between 1995 and 2002 in 41 patients with recurrent LGSC. The study concluded that secondary cytoreduction is beneficial.[
66 ][Level of evidence C3] The median time from primary tumor debulking until secondary cytoreduction was 33.2 months.- Thirty-two patients (78%) had gross residual disease at completion of secondary cytoreduction.
- Patients with no gross residual disease after secondary cytoreduction had a median progression-free survival (PFS) of 60.3 months compared with 10.7 months for those with gross residual disease (P = .008).
- The overall survival (OS) from secondary cytoreduction surgery was 93.6 months for those with no gross residual disease after surgery compared with 45.8 months for those with gross disease (P = .04).
Targeted therapies
As LGSC is relatively chemoresistant, attention has focused on targeted therapies.
- Anastrozole: Estrogen receptor (ER) expression is noted in most LGSCs and is a potential target. The PARAGON phase II basket trial evaluated the activity of anastrozole in patients with ER-positive tumors, including 36 patients with recurrent LGSC.[
77 ][Level of evidence C3]- The trial demonstrated clinical benefit in 61% of patients at 6 months and 34% of patients at 12 months. The study did not only look at patients with LGSC.
- The median duration of clinical benefit was 9.5 months.
- Trametinib: Trametinib is a selective, reversible, allosteric inhibitor of MEK1/MEK2. A recent international, randomized, multicenter, phase II/III trial evaluated trametinib in patients with LGSC. An unlimited number of prior therapies, including chemotherapy or hormone therapy, was allowed. Patients had to have received at least one prior platinum-based regimen, but not all five standard-of-care drugs. Patients received either oral trametinib (2 mg once daily) or the standard of care with physician's choice of chemotherapy (paclitaxel, pegylated liposomal doxorubicin, topotecan, oral letrozole, or oral tamoxifen).[
88 ][Level of evidence B1]- The median PFS was 13.0 months (95% confidence interval [CI], 9.9–15.0) in the trametinib group and 7.5 months (95% CI, 5.6–9.9) in the standard-of-care group (hazard ratio [HR], 0.48; 95% CI, 0.36–0.64; one-sided P < .0001).
- A post hoc analysis was performed on a subgroup of 87 patients who were preplanned to receive letrozole if randomly assigned to the standard-of-care group. The PFS was 15.0 months (95% CI, 7.7–23.1) in the trametinib group and 10.6 months (95% CI, 6.5–12.8) in the letrozole group (HR, 0.58; 95% CI, 0.36–0.95; one-sided P = .0085).
- The overall response rate was 26% (34 of 130 patients) in the trametinib group. An additional 59% of patients (77 of 130) had stable disease for at least 8 weeks.
- The median OS was 37.6 months (95% CI, 32.0–not evaluable) in the trametinib group and 29.2 months (23.5–51.6) in the standard-of-care group (HRdeath, 0.76; 95% CI, 0.51–1.12; one-sided P = .056).
- There was no evidence that BRAF, KRAS, or NRAS mutation status predicted PFS.
- Binimetinib: MILO/ENGOT-ov11 (NCT01849874) was a phase III trial of binimetinib, a small molecule inhibitor of MEK1/MEK2. The trial included 303 patients with recurrent LGSC who had received one to three prior lines of chemotherapy. Patients were randomly assigned in a 2:1 ratio to receive either binimetinib (45 mg orally twice daily) or physician's choice of chemotherapy (pegylated liposomal doxorubicin, paclitaxel, or topotecan).[
99 ]- The median PFS was 9.1 months (95% CI, 7.3–11.3) for patients who received binimetinib and 10.6 months (95% CI, 9.2–14.5) for patients who received chemotherapy. The study closed early according to the prespecified futility boundary.
- The overall response rate was 16% for patients who received binimetinib (including 32 patients with a complete/partial response), and 13% for patients who received chemotherapy (including 13 patients with a complete/partial response).
- The median duration of response was 8.1 months (range, 0.03 to ≥ 12.0) for patients who received binimetinib and 6.7 months (range, 0.03 to ≥ 9.7) for patients who received chemotherapy.
- The OS was 25.3 months for patients who received binimetinib and 20.8 months for patients who received chemotherapy.
- OS was similar and viewed as no better or worse than chemotherapy.
- A post hoc analysis was conducted on tumor molecular testing samples from 215 patients. A KRAS mutation was seen in 32% to 34% of patients. The analysis showed that KRAS mutation was associated with response to treatment with binimetinib (odds ratio, 3.4; 95% CI, 1.53–7.66; unadjusted P = .003) and with prolonged PFS in patients treated with binimetinib (median PFS: KRAS mutant, 17.7 months [95% CI, 12–not reached]; KRAS wild-type, 10.8 months [95% CI, 5.5–16.7]; P = .006).
- Bevacizumab: Bevacizumab is an anti-VEGFA monoclonal antibody. A single institution enrolled 13 patients with LGSC and 4 patients with borderline tumors. Two patients received bevacizumab as a single agent while the other patients received bevacizumab in combination with chemotherapy (i.e., paclitaxel, topotecan, oral cyclophosphamide, gemcitabine, or gemcitabine and carboplatin).[
1010 ][Level of evidence C3]- After a median duration of 23 weeks (range, 6–79.4), there were no patients with a complete response and six patients with a partial response (five of whom had received concurrent paclitaxel, and one who received concurrent gemcitabine). The overall response rate was 40% among all evaluable patients, and 55% among the subgroup of LGSC patients.
- Ribociclib and letrozole: GOG-3026 (NCT03673124) was a phase II trial evaluating the combination of ribociclib and letrozole in patients with recurrent LGSC. Patients received ribociclib, a CDK4/6 inhibitor, at 600 mg daily on a 3-weeks-on/1-week-off schedule, plus 2.5 mg of oral letrozole once daily for a 28-day cycle.[
1111 ]- The overall response rate was 23% (90% CI, 13.4%–35.1%) and the clinical benefit rate was 97% (90% CI, 67.2%–88.2%)
- The median duration of response was 19.1 months. The median PFS was 19.1 months, and the median OS was not reached.
- Selumetinib: Selumetinib is a selective small molecule inhibitor of MEK1/MEK2. A phase II study included 52 patients with recurrent LGSC who received 50 mg of selumetinib twice daily until progression.[
1212 ][Level of evidence C3]- There were 8 patients (15%) with complete responses, 7 patients (13%) with partial responses, and 34 patients (65%) with stable disease.
- The median time to response was 4.8 months, and the median duration of response was 10.5 months.
- The median PFS was 11 months, and 63% of patients had a PFS of more than 6 months.
- Responses were irrespective of BRAF or KRAS mutations.
- Imatinib: LGSC has a high expression of PDGFR-β and BCR::ABL. A phase II study evaluated imatinib mesylate in patients with platinum-resistant recurrent LGSC. The trial enrolled 13 patients who had received at least four prior lines of platinum- and/or taxane-containing chemotherapy and had been screened for a targeted biomarker (i.e., c-kit, PDGFR-β, or BCR::ABL). Patients received 600 mg of imatinib mesylate daily for 6 weeks.[
1313 ]- There was no evidence of efficacy in patients who received imatinib mesylate despite high expression of PDGFR-β.
References:
- Gershenson DM: Low-grade serous carcinoma of the ovary or peritoneum. Ann Oncol 27 (Suppl 1): i45-i49, 2016.
- Fader AN, Java J, Krivak TC, et al.: The prognostic significance of pre- and post-treatment CA-125 in grade 1 serous ovarian carcinoma: a gynecologic Oncology Group study. Gynecol Oncol 132 (3): 560-5, 2014.
- Norquist BM, Brady MF, Harrell MI, et al.: Mutations in Homologous Recombination Genes and Outcomes in Ovarian Carcinoma Patients in GOG 218: An NRG Oncology/Gynecologic Oncology Group Study. Clin Cancer Res 24 (4): 777-783, 2018.
- Gershenson DM, Sun CC, Lu KH, et al.: Clinical behavior of stage II-IV low-grade serous carcinoma of the ovary. Obstet Gynecol 108 (2): 361-8, 2006.
- Scott SA, Llaurado Fernandez M, Kim H, et al.: Low-grade serous carcinoma (LGSC): A Canadian multicenter review of practice patterns and patient outcomes. Gynecol Oncol 157 (1): 36-45, 2020.
- Crane EK, Sun CC, Ramirez PT, et al.: The role of secondary cytoreduction in low-grade serous ovarian cancer or peritoneal cancer. Gynecol Oncol 136 (1): 25-9, 2015.
- Tang M, O'Connell RL, Amant F, et al.: PARAGON: A Phase II study of anastrozole in patients with estrogen receptor-positive recurrent/metastatic low-grade ovarian cancers and serous borderline ovarian tumors. Gynecol Oncol 154 (3): 531-538, 2019.
- Gershenson DM, Miller A, Brady WE, et al.: Trametinib versus standard of care in patients with recurrent low-grade serous ovarian cancer (GOG 281/LOGS): an international, randomised, open-label, multicentre, phase 2/3 trial. Lancet 399 (10324): 541-553, 2022.
- Monk BJ, Grisham RN, Banerjee S, et al.: MILO/ENGOT-ov11: Binimetinib Versus Physician's Choice Chemotherapy in Recurrent or Persistent Low-Grade Serous Carcinomas of the Ovary, Fallopian Tube, or Primary Peritoneum. J Clin Oncol 38 (32): 3753-3762, 2020.
- Grisham RN, Iyer G, Sala E, et al.: Bevacizumab shows activity in patients with low-grade serous ovarian and primary peritoneal cancer. Int J Gynecol Cancer 24 (6): 1010-4, 2014.
- Slomovitz B, Deng W, Killion J, et al.: GOG 3026 A phase II trial of letrozole + ribociclib in women with recurrent low-grade serous carcinoma of the ovary, fallopian tube or peritoneum: A GOG foundation study. [Abstract] Gynecol Oncol 176 (Suppl 1): A-001, S2, 2023.
- Farley J, Brady WE, Vathipadiekal V, et al.: Selumetinib in women with recurrent low-grade serous carcinoma of the ovary or peritoneum: an open-label, single-arm, phase 2 study. Lancet Oncol 14 (2): 134-40, 2013.
- Noguera IR, Sun CC, Broaddus RR, et al.: Phase II trial of imatinib mesylate in patients with recurrent platinum- and taxane-resistant low-grade serous carcinoma of the ovary, peritoneum, or fallopian tube. Gynecol Oncol 125 (3): 640-5, 2012.
Latest Updates to This Summary (05 / 01 / 2024)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
This summary was renamed from Ovarian Low Malignant Potential Tumors Treatment.
This summary was extensively revised.
This summary is written and maintained by the
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of ovarian borderline tumors. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Ovarian Borderline Tumors Treatment are:
- Olga T. Filippova, MD (Memorial Sloan-Kettering Cancer Center )
- Marina Stasenko, MD (New York University Medical Center)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Adult Treatment Editorial Board uses a
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as "NCI's PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary]."
The preferred citation for this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Ovarian Borderline Tumors Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at:
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either "standard" or "under clinical evaluation." These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our
Last Revised: 2024-05-01
This information does not replace the advice of a doctor. Ignite Healthwise, LLC, disclaims any warranty or liability for your use of this information. Your use of this information means that you agree to the
Healthwise, Healthwise for every health decision, and the Healthwise logo are trademarks of Ignite Healthwise, LLC.
Page Footer
I want to...
Audiences
Secure Member Sites
The Cigna Group Information
Disclaimer
Individual and family medical and dental insurance plans are insured by Cigna Health and Life Insurance Company (CHLIC), Cigna HealthCare of Arizona, Inc., Cigna HealthCare of Illinois, Inc., Cigna HealthCare of Georgia, Inc., Cigna HealthCare of North Carolina, Inc., Cigna HealthCare of South Carolina, Inc., and Cigna HealthCare of Texas, Inc. Group health insurance and health benefit plans are insured or administered by CHLIC, Connecticut General Life Insurance Company (CGLIC), or their affiliates (see
All insurance policies and group benefit plans contain exclusions and limitations. For availability, costs and complete details of coverage, contact a licensed agent or Cigna sales representative. This website is not intended for residents of New Mexico.